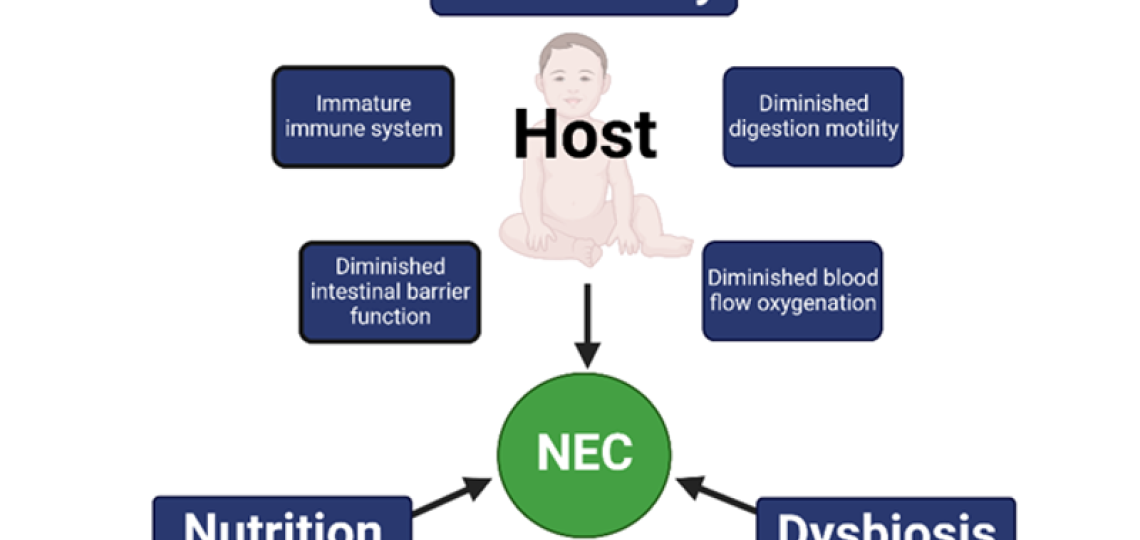
Another important research area uses our clinically relevant, premature piglet model of NEC to establish the critical elements of pathogenesis and strategies for prevention. Our previous studies with long-time collaborator, Per Sangild, Ph.D. at University of Copenhagen established that prematurity, microbial colonization and diet are necessary elements in the pathogenesis of NEC. The Figure below shows the “NEC triad” of the major risk factors for NEC including prematurity, gut dysbiosis and nutrition.
More recent studies by BCM graduate student, Lee Call, Ph.D. have shown that malabsorption of carbohydrates, namely maltodextrins, in infant formula can trigger bacterial overgrowth and promote the onset of NEC. Current studies led by Valeria Melendez Hebib are aimed at establishing how the components of human milk and infant formula shape the microbiome-metabolomic interaction with the host immune function and prevents NEC.
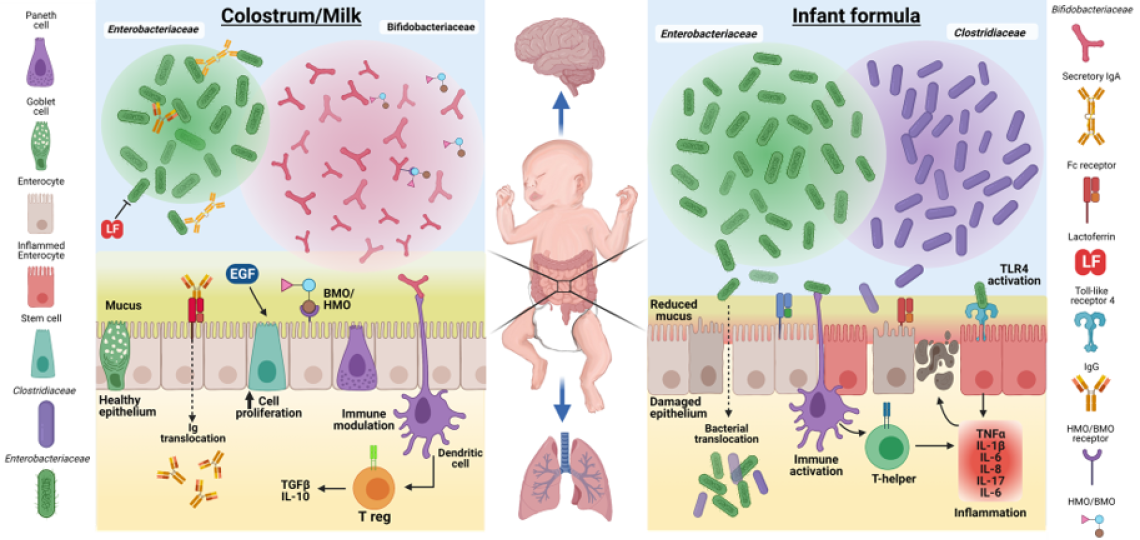
The figure illustrates some of the proposed functional effects of maternal colostrum/milk bioactive factors on the dominant gut microbiota communities, mucosal epithelium, and immune cells, relative to formula diets. Enterobacteriaceae, Bifidobacteraceae, and Clostridiaceae families represent common bacteria groups found in the developing neonatal gut. The bacterial community composition can be affected by maternal milk factors, such as Igs (IgA and IgG), LF, bovine and human oligosaccharides (BMO/HMO), and a number of growth factors (e.g., EGF). Igs and LF have antimicrobial properties that function to limit epithelial inflammation and apoptosis resulting from activation of Toll-like receptor 4 (TLR4) by Enterobacteriaceae. BMO/HMO may serve as a substrate for growth and colonization of Bifidobacteraceae, a family of bacteria associated with gut health. The figure also illustrates that key peripheral organs, including the brain and lungs, directly or indirectly may be impacted by colostrum/milk-induced improved gut microbial activity and gut mucosal immune defense.