DBS in Obsessive-Compulsive Disorder
Obsessive-compulsive disorder (OCD) has a lifetime prevalence of 2-3% and is a major cause of global disability. OCD is characterized by obsessions (i.e. unwanted intrusive thoughts) and compulsions (i.e. repetitive ritualistic behaviors), and exemplifies a pathologically avoidant phenotype where greater degrees of avoidance are associated with greater symptom severity and treatment resistance. In the 20-40% of OCD patients that are treatment refractory, deep brain stimulation (DBS) of the ventral capsule and ventral striatum (VC/VS) is an effective therapy that achieves significant benefit in 66% of patients.
While VC/VS DBS does not acutely relieve OCD symptoms, DBS often produces a "pro-approach" response including an increase in talkativeness, a desire to engage in activities, extroversion, and affiliative behavior. Our hypothesis is that VC/VS DBS achieves eventual benefit in OCD by providing the “boost” in pro-approach behavior that allows individuals to overcome their pathological fear-based avoidance. Thus, we focus on the approach-avoidance axis as one of the critical neurobehavioral axes underlying the pathophysiology of OCD. Our overall goal is to develop a mechanistic understanding of DBS for OCD by rigorously investigating the relationship between clinical symptoms, behaviors (i.e., approachful vs. avoidant), and neurophysiology.
In OCD patients with recording-capable DBS devices, we are collecting a broad array of neurobehavioral data in the clinic and the home. These data will allow us to use intracranial recordings from the ventral striatum to inform and validate the approach-avoidance framework and investigate the degree of explanatory power this framework has for OCD. By understanding the therapeutic mechanism driving OCD symptom improvement after VC/VS DBS, we will pave the way for rigorous, data-driven neuromodulatory strategies to regulate behavior and ameliorate symptoms in other disorders characterized by dysregulated approach-avoidance behavior.
Team leads: Tommy Liu, Zain Naqvi, Holden Bentley, Kasra Mansourian
Collaborators: Sameer Sheth, Wayne Goodman, Eric Storch, Jeffrey Herron, Ankit Patel, Benjamin Hayden

DBS in Treatment-Resistant Bipolar Disorder
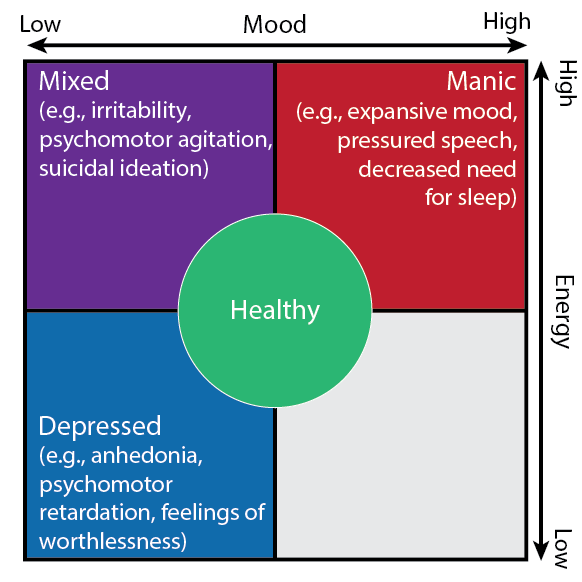
Bipolar Disorder (BD) is a recurrent neuropsychiatric disorder characterized by wide fluctuations in mood, energy, and activity. Although the hallmark of BD is mania, depressive episodes dominate the longitudinal course and account for a disproportionate degree of morbidity and mortality. About 25% of patients with BD meet criteria for treatment-resistant bipolar depression (TRBD). Given the gravity of the illness and paucity of available therapies, there is a large unmet need for more effective interventions. We are conducting an early feasibility study to advance the therapy and neurobehavioral monitoring of TRBD with deep brain stimulation (DBS) targeting the ventral capsule/ventral striatum (VC/VS) using a recording-capable DBS system. We will leverage a smart phone-based software platform paired with the device’s chronic sensing capability to record local field potentials (LFPs) time-locked with remote, high-density measures of natural behavior (e.g., from wearables). Our goal is to detect relevant behavioral states including both depression and mania and transitions between these states, including high risk “mixed” features. Real-time, remote detection of affective states via both behavioral and neural data monitoring will enable new interventional strategies to shift behavior away from pathological behavioral states towards healthier ones, contributing to the long-term stability of patients.
Team leads: Thomas Kutcher, Tomasz Fraczek, Yewen Zhou
Collaborators: Wayne Goodman, Sameer Sheth, Jeffrey Herron, Benjamin Hayden, Nora Vanegas, Sarah Heilbronner, Ashutosh Sabharwal
DBS in Parkinson's Disease
While Parkinson’s Disease (PD) is typically defined by motor features, non-motor characteristics are highly prevalent and are often as debilitating as motor issues. This evolving understanding of PD symptom heterogeneity has led to a conception of the disease as the “quintessential neuropsychiatric disorder” and to an increased focus on the evaluation of global functioning, a parameter acknowledged by the World Health Organization as central to assessing health and disability. The primary contributors to diminished global functioning and quality of life in PD are dysfunction in mobility, mood, socialization, and sleep. The growing recognition of the impact of non-motor contributors to global functioning contrasts with the historical focus on treatments for motor symptoms, whether pharmacologically or with deep brain stimulation (DBS). This disparity demands a rebalancing of research efforts toward a more comprehensive emphasis across all the primary contributors of global functioning in PD. Current assessment methods lack the ability to reliably measure both motor and non-motor symptoms, often relying on infrequent clinical evaluations and subjective reporting. Leveraging advancements in wearable technology and the availability of DBS sensing capabilities (i.e., local field potentials), our project aims to develop a software platform for continuous neurobehavioral monitoring of the most critical contributors to global functioning in PD (mobility, mood, socialization, and sleep), in naturalistic settings. An improved understanding of the neural and behavioral mechanisms underlying global functioning in PD will allow for improved, targeted, individualized treatment approaches.
Team Leads: Sai Chamarthi, Zain Naqvi
Collaborators: Nora Vanegas, Sameer Sheth, Jeffrey Herron, Ashutosh Sabharwal, Sarah Heilbronner, Benjamin Hayden








