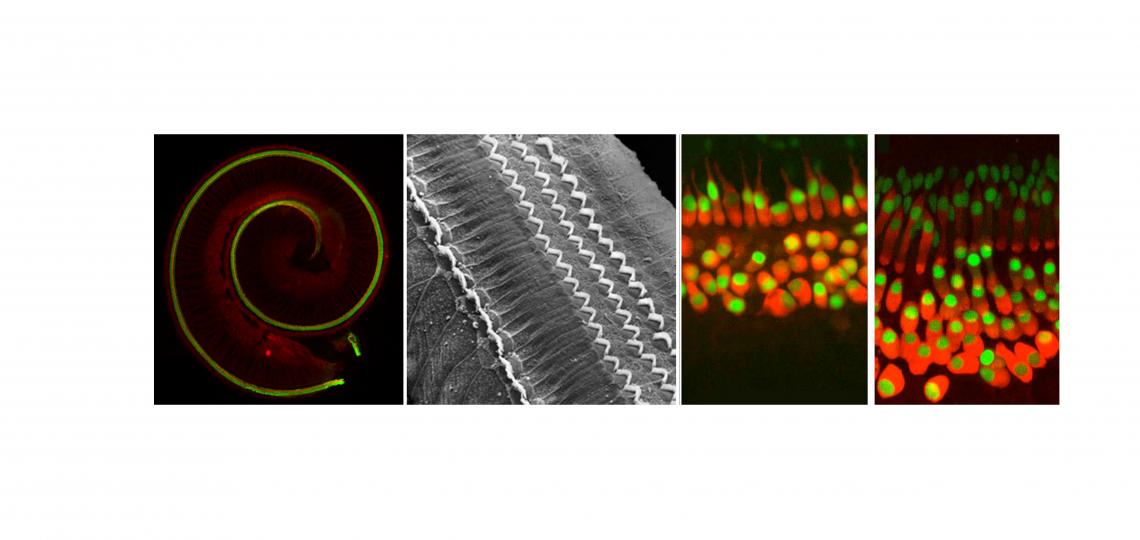
How Does the Cochlea Develop?
The organ of Corti – the hearing organ of the inner ear - forms from a ribbon of progenitor cells running the length of the cochlear duct, termed the prosensory domain. Starting at about embryonic day 12 in the mouse, the prosensory domain exits the cell cycle and expresses the cell cycle inhibitor p27kip1. Over the next two days, this wave of cell cycle exit spreads from the apex of the cochlea to the base. The post-mitotic prosensory domain then begins to differentiate into hair cells and supporting cells, starting near the base of the cochlea and progressing to the apex over several days. In other words, the first cells to exit the cell cycle are the last ones to differentiate! The signals that drive these two mysterious developmental gradients are largely unknown.
How does the mature cochlear duct develop? The embryonic prosensory domain – destined to give rise to the organ of Corti - is bounded on its neural side by a thickened epithelial structure known as Kölliker’s organ, which will eventually form the inner sulcus, and on its abneural side by the future outer sulcus composed of Hensen’s and Claudius’ cells. We discovered that BMP signaling forms a gradient across the abneural-neural axis of the cochlear duct, with highest levels of signaling in the future outer sulcus, lowest levels in Kölliker’s organ, and intermediate levels being seen in the prosensory domain. We suggest that the cochlea is patterned by a morphogen gradient of BMP signaling, and that the three components of the cochlea duct – Kölliker’s organ (inner sulcus), the prosensory domain (organ of Corti) and the outer sulcus are specified by increasing concentrations of BMPs. In the absence of BMP signaling, the cochlear duct develops exclusively with the identity of Kölliker’s organ. We are continuing to try and understand how BMP acts as a morphogen to specify different cochlear fates at different concentrations using transgenic and knockout mice and single cell RNA-seq
Notch signaling has been proposed to play a variety of roles in inner ear development, including sharpening the boundary of the otic placode and epidermis, regulating the production of neurons from the otocyst and inducing the sensory patches of the inner ear by direct induction. However, it has proven hard to completely and specifically eliminate Notch signaling in the inner ear, and we are now trying to do this with conditional inactivation of multiple Notch receptors.
We recently found that Notch signaling is at least twice during differentiation of the organ of Corti. First, a low level of Notch signaling, which is exquisitely sensitive to perturbation, sets the boundary of the organ of Corti. Subsequently, much higher levels of Notch signaling are used to distinguish between sensory hair cells and their neighboring supporting cells. This is one of the first examples where regulating Notch signaling strength leads to very different developmental outcomes in the same tissue. We have found that part of this fine-tuned regulation is carried out by two members of the Fringe family of glycosyltransferases that can modify Notch receptors and ligands. Interestingly, expression of Lunatic Fringe and Manic Fringe converge precisely at the boundary of the organ of Corti, and mutation of both genes causes disruptions to this boundary in a manner reminiscent of Fringe function in Drosophila imaginal discs.
Gu, R., Brown, R.M., Hsu, C.-W., Crowder, A., Piazza, V.G., Vadakkan, T.J., Cai, T., Dickinson, M.E. and Groves, A.K. (2016). Lineage tracing of Sox2-expressing progenitor cells in the mouse inner ear reveals a broad contribution to non-sensory tissues and insights into the origin of the organ of Corti. Developmental Biology, 414, 72-84.
Basch, M.L., Brown, R.M., Jen, H.-I and Groves, A.K. (2016). Where hearing starts: The development of the mammalian cochlea. J. Anatomy, 228, 233-254.
Raft, S. and Groves, A.K. (2015). Segregating neural and mechanosensory fates at the developing ear: patterning, signaling, and transcriptional control. Cell and Tissue Research 359, 315-332.
Cai, T. and Groves, A.K. (2015). The role of Atonal factors in mechanosensory cell specification and function. Cellular Neurobiology, in press.
Moayedi, Y., Basch, M.L., Pacheco, N., Gao, S.G., Harrison, W., Xioa, N, Oghalai, J.S., Overbeek, P.A., Mardon G., and Groves A.K. (2014). The splicing factor Sfswap regulates growth and patterning of inner ear sensory organs. PLoS Genetics 10(1): e1004055
Cai, T, Seymour, M.L., Zhang, H., Pereira, F. A. and Groves, A.K. (2013). Conditional deletion of Atoh1 reveals distinct critical periods for survival and function of hair cells in the organ of Corti. J. Neuroscience 33, 10110-10122.
Groves, A.K., Zhang, K.D., and Fekete, D.M. (2013). The genetics of hair cell development and regeneration. Ann. Rev. Neuroscience, 36, 361-381.
Jarman, A.P. and Groves, A.K. (2013). The role of atonal transcription factors in the development of mechanosensitive cells. Seminars in Cell and Developmental Biology, 24, 438-447
Groves, A.K. and Fekete, D.M. (2012). Shaping sound in space: The regulation of inner ear patterning.Development 139, 245-257.
Basch, M.L., Ohyama, T., Segil, N. and Groves, A.K. (2011). Canonical Notch signaling is not necessary for prosensory induction in the mouse cochlea: Insights from a conditional mutant of RBPjk. Journal of Neuroscience 31, 8046-8058
Ohyama, T., Basch, M.L., Mishina, Y., Lyons, K., Segil, N., and Groves, A.K. (2010). BMP signaling is necessary for patterning the sensory and non-sensory regions of the developing mammalian cochlea. Journal of Neuroscience 30, 15044-15051.
Doetzlhofer, A., Basch, M.L., Ohyama, T., Gessler, M., Groves, A.K. and Segil, N. (2009). Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti.Developmental Cell 16, 58-69.
Raft, S., Koundakjian, E.J., Quinones, H., Jayasena, C.S., Goodrich, L.V., Johnson, J.E., Segil, N. and Groves, A.K. (2007). Cross-regulation of Ngn1 and Math1 coordinates the production of neurons and sensory hair cells during inner ear development. Development 134, 4405-4415.








