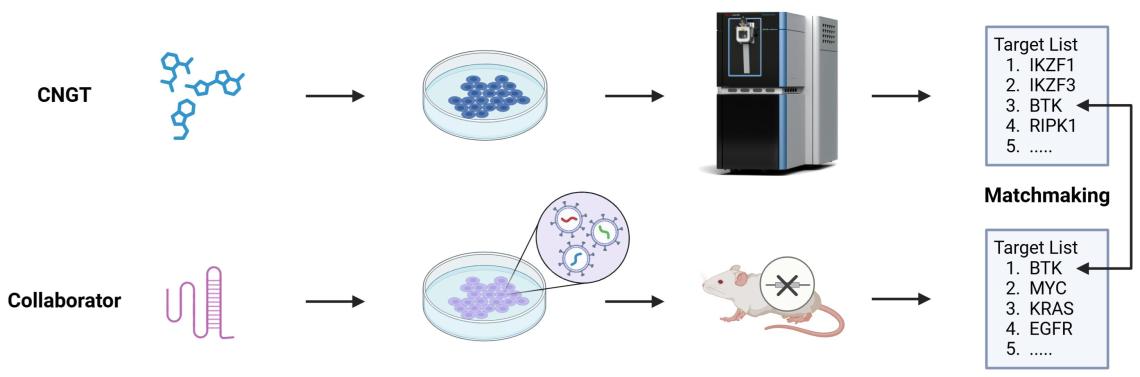
Drug discovery is a costly and complex process, particularly in the early stages of identifying initial hits. To mitigate this challenge and lower the entry barrier for biologists, CNGT employs a strategic matchmaking approach.
Matchmaking
By leveraging state-of-the-art proteomics technologies, CNGT conducts comprehensive, target-agnostic screens across the entire proteome to identify a curated list of proteins that demonstrate potential for small molecule targeting. Simultaneously, collaborators use functional genomics and other methods to pinpoint and confirm drug targets both in vitro and in vivo. The resulting target list is shared with CNGT members, offering a significant asset for their research. If a member finds their target of interest on the list, CNGT will supply the relevant compound to help validate its mechanism of action. Upon confirmation that the compound successfully engages the intended target, CNGT offers the opportunity for collaborative co-development of the compound series, focusing on optimizing its drug-like properties.
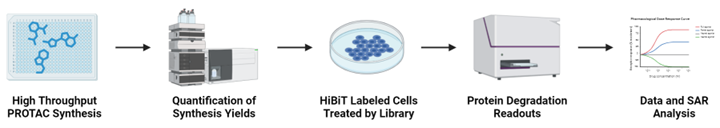
High Throughput PROTAC Direct-to-Biology (D2B) Screen
If collaborators have biologically validated targets for protein degradation, CNGT can carry out high-throughput PROTAC synthesis in 384-well plates for targets with identified ligands. The library synthesis yields are measured using LC-MS/MS. The crude PROTAC library is then directly applied to cells expressing the HiBiT-tagged protein of interest, bypassing purification. Protein levels are monitored through changes in bioluminescence signals. The resulting dose-response curves and structure-activity relationships are subsequently analyzed.