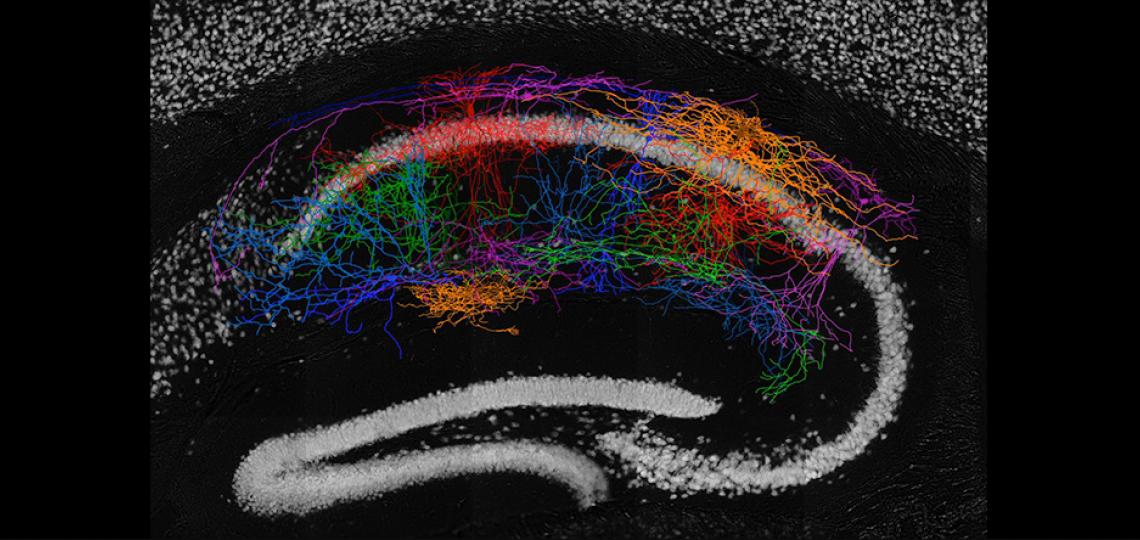
Morphological Reconstruction of the CA1 Neurons
Morphological reconstruction of the CA1 neurons in the hippocampus revealed the high diversity of interneuron types in mouse hippocampus CA1 area.
Decipher the Building Blocks of Mouse Hippocampus CA1
Given its essential role in learning and memory and simply organized anatomy, the hippocampus is one of the most heavily studied regions of the mammalian brain. While a trisynaptic circuit organization (dentate gyrus-to-CA3-to-CA1) among the hippocampal principal cells has long been established, a complete census of its GABAergic cell types and their connectivity still remains elusive. We are combining high-throughput multi-cell patch recordings, morphological recovery, single-cell RNA sequencing, and state-of-the-art machine learning to catalogue the inhibitory interneuron types in the mouse hippocampus CA1 and determine the canonical connectivity rules between them and pyramidal cells. The goal of the study is to provide a detailed blueprint of the cell type-specific wiring diagram of the local circuit of CA1, which will serve a critical framework to decipher the role that local CA1 circuits play in computations such as memory formation.
Decipher the Building Blocks of Monkey Prefrontal Cortex
The dorsolateral prefrontal cortex (DLPFC) of the primate brain is the most evolutionarily developed brain region that supports complex cognitive processes characteristic of primates, such as reasoning, planning, and abstract thinking. However, we know little about the constituent cell types comprising DLPFC circuit, how each cell type connects each other to form a functional circuit, and what circuit components specific to this circuit endow it with superb computational capabilities for complex cognitive processes. To fill in this knowledge gap, we are using a cost-effective, interdisciplinary approach, including multi-cell patch recordings, single-cell RNA sequencing, and machine learning , to identify all the constituent cell types comprising macaque DLPFC and decipher their connectivity rules, with emphasis on highly diverse GABAergic interneurons. The project have three main goals to achieve: 1) generate the taxonomy of transcriptomic cell types that comprise DLPFC and understand the electrophsiology and morphology of each neuronal cell type; 3) derive and verify the transcriptomic signatures of each cell type; 3) mapping the connection patterns of each cell type.
Identify "Seizure-Causing" Cell Type in Surgically Resected Tissue from Tuberous Sclerosis Patients
Tuberous sclerosis complex (TSC) is complex genetic disorder often associated with pharmacoresistant seizures, autism, and intellectual disability, characterized by the presence of anatomical malformations in brain known as cortical tubers (CTs) as well as tumors in other organs. Despite the clear involvement of mutations in either TSC1 or TSC2 gene in TSC, it remains to be elucidated how TSC1/TSC2 deficiency ultimately leads to pharmacoresistant seizures and other cognitive comorbidities. It is generally believed that seizures in TSC arise from CT structure, and preoperative localization and subsequent surgical removal of seizure-causing CTs often results in seizure freedom. CTs are characterized by the presence of bizarre-looking brain cells that are not present in the normal brain structure, which has led to the speculation that these cells are the “seizure-causing” cell types.
However, whether or not these abnormal cells are “seizure-causing” appears to an intractable question in the field, since rodent models do not develop the abnormal cell types and structure as seen in human TSC patients. We are thus taking advantage of a wealth of surgically resected CT tissue at our institutes, and apply the sensitive, high-throughput approach pioneered in basic research directly into the seizure-causing human CT tissue to address these seemingly intractable questions. We perform a multi-modal analysis of these cell types in CT tissue, particularly on how these cell types electrochemically interact with other cells, which may lead to the identification and characterization of the potential “seizure-causing” cell type in term of physiology, morphology, connectivity and whole-genome gene expression profile.

Simultaneous 11-cell Patch-clamp Recordings
Simultaneous 11-cell patch-clamp recordings were made on cortical neurons from surgically resected human brain tissue, and their morphology was recovered, and connectivity was tested between any of neurons
Identify "Seizure-Causing" Cell Type in Surgically Resected Tissue from Epilepsy Patients
Mesial temporal lobe epilepsy (mTLE) is the most common focal epilepsy, associated with cognitive comorbidities, and refractory to medication in over 80 percent of patients. Despite lacking a clear etiology, mTLE is generally associated with hippocampal sclerosis (HS), the most common ictogenic lesion encountered in patients with epilepsy. While the CA3 and CA1 regions of the hippocampus in HS are damaged or even absent, the dentate gyrus (DG) is largely spared and believed to be a critical neural substrate for seizure generation and expression. It is well documented that DG in mTLE exhibits profound circuit reorganization that may disrupt the canonical wiring principles, or even change the complement of cell types specific to its local circuit.Among these, the most prominent are the emergence of an atypical morphotype of dentate granule cells (DGCs with a basal dendrite in hilus (HBD), DGC-HBD, different from typical DGCs) and the extensive sprouting of DGC mossy fibers, which are believed to form aberrant recurrent excitatory connections among DGCs to promote seizures.
However, it still remains controversial as to whether these aberrant circuit components form functional connections, and if they do, what functional roles of these aberrant connections may play in seizure generations. To directly address these questions, we take advantage of the wealth of live ictogenic human brain tissue resected from mTLE patients in the Texas Medical Center to prepare human brain slices for electrophysiology. By leveraging a high-throughput multi-patching method (up to 12 cells) combined with morphology recovery, and high viability of human brain slices (alive >3 days), we are able to extensively examine how DGCs connect each other using these human mTLE samples, despite their low availability. In addition, we are applying single-cell RNA-sequencing on surgerical tissue samples to understand the relationship between aberrant neurogenesis on human DG, abnormal cell types and circuit misorganization.
We expect our results directly from human patients will help settle the controversies in the field, and uncover a cohesive picture of how the DG circuit is reorganized to support the initiation and propagation of seizures, which may provide new strategies for mTLE treatments.
Development of Aberrant Cortical Interneuron Circuitry in Three Genetic Mouse Models of Absence Epilepsy
Generalized spike-wave (SW) absence seizures are the most common seizure disorder in children and thought to be exclusively of genetic origin. It is believed that inherited molecular defects interfere with neural development and lead to dysfunctional neural circuits that can pathologically initiate, maintain, and propagate hypersynchronous and hyperexcitable neural activity manifesting as spontaneous, recurrent SW seizures. While over 20 genes are discovered and studied in SW epilepsies, it is still unclear how each genetic lesion impairs cortical circuit development and ultimately results in a seizure-prone neural circuit.
Recently, as we have begun to understand the canonical wiring principles of cortical circuits at the level of cell type, it has become possible to ask how disruption of these canonical networks is responsible for initiating seizure activity and impairing cognitive function in SW epilepsies. Since the SW seizure phenotype can be very similar despite disparate genetic etiologies, a stereotypical circuit deficit may develop from inherited molecular defects to favor seizure onset at predictable developmental time-points.
To test this hypothesis, we are taking advantage of three mouse models of absence epilepsy, stargazer (stg, Cacng2/TARP), tottering (tg, Cacna1a/PQ Ca2+ channel alpha subunit) and Gabrg2 mutant mice (Gabrg2+/-, Gabrg2/ GABAAR γ2 subunit), which harbor monogenic mutations in three unrelated genes but have a similar SW phenotype, and conduct a comprehensive microcircuit comparisons among distinct genotypes at the level of cell types and their connections along the course of seizure development. Our data suggest a common circuit deficit underlying the expression of SW seizures depsite disparate genetic etiologies. The potential causative roles of identified circuit deficits in SW expression will be further tested via in silico network modeling and an in vivo chemicogenetic approach.








