Our research focuses on two broadly interrelated areas: ligandomics technology for drug target discovery and biological drug development for disease-targeting therapy. We created the first technology of ligandomics that led to multiple ongoing drug development projects. The goal of our ligandomics project is to develop an advanced ligand research tool that is capable of systematically mapping disease-selective ligands for in-depth understanding of disease mechanisms and facilitate drug target discovery. The goal of our drug development program is to independently validate disease-selective ligands as drug targets, generate neutralizing antibody to inhibit detrimental ligands, and develop therapeutic antibodies toward clinical translation.
Ligandomics: A Paradigm Shift in Drug Target Discovery
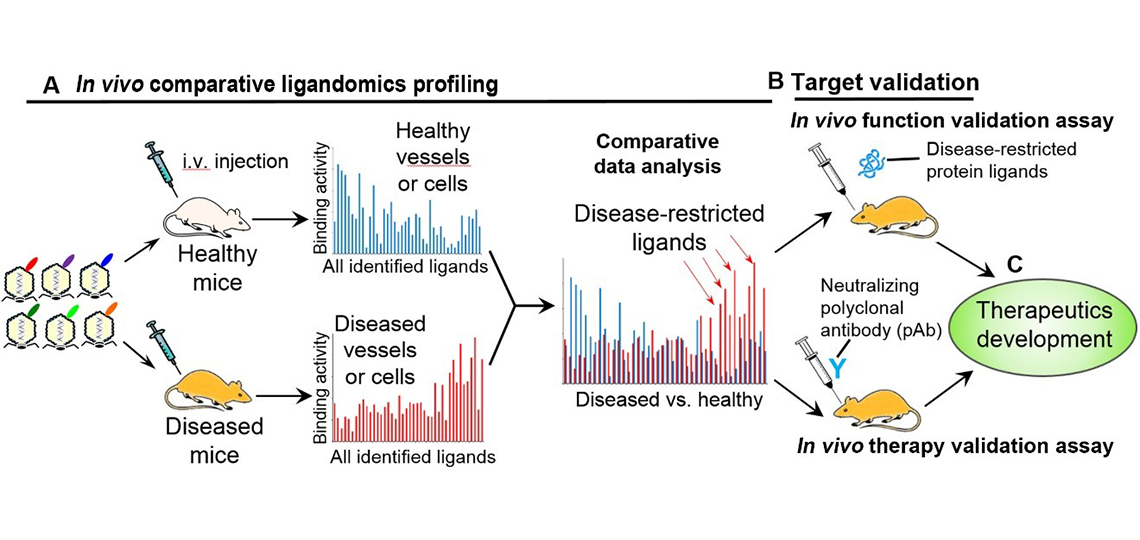
General process for ligandomics to discover disease-selective drug targets and develop novel therapies. (A) Comparative ligandomics profiling to systematically map disease-selective ligands. (B) Function-first and/or therapy-first ligandomics approaches to independently characterize functional activity, disease selectivity, pathogenic role and therapeutic potential of identified ligands. (C) Drug development (PubMed).
Cell-cell communication is fundamental to the development and homeostasis of multicellular organisms. Of all intercellular regulations, cell surface ligand-receptor interactions are one of the most important signaling conduits to regulate a broad spectrum of cellular functions, ranging from stem cell niches and morphogenesis to disease and therapy.
Autocrine and paracrine factors connect ligand-secreting and responding cells, thereby holding the key to mapping precise pathways of intercellular crosstalk.
Ligand-receptor interactions can be amplified and ramified through multiple layers of intracellular signaling cascades to regulate a broad spectrum of cellular functions.
Therefore, pharmacological interventions directed at such early cell surface signaling events may be therapeutically more effective than interventions at locations further downstream. Indeed, 40% of all FDA-approved drugs target cell surface receptors.
However, only a limited number of extracellular ligands have been successfully exploited with approved drugs. This is probably because secreted ligands are more elusive and are traditionally discovered on a case-by-case basis with inherent technical challenges, whereas the human plasma membrane receptome has been fully mapped based on receptor transmembrane domains and cell surface expression.
Our ligandomics is the first technology to globally map cell-wide ligands, and our quantitative ligandomics can simultaneously quantify the binding or functional activity of entire ligandomes. Importantly, comparative ligandomics is capable of systematically profiling disease-selective ligands as high-quality drug targets to develop disease-targeting therapies with minimal side effects on healthy cells.
Our function-first and therapy-first ligandomics approaches are designed to independently characterize functional activity, disease selectivity, pathogenic role and therapeutic potential of identified ligands.
We are developing advanced ligandomics technology to further improve sensitivity, reliability and broad applicability of this new technology that will facilitate disease mechanism research and drug target discovery for both ocular and non-ocular diseases.
Our ligandomics project has led to issued patents. A startup biotech company is funded by a NIH business grant to further develop ligandomics technology.
Next-Generation Disease-Targeting Anti-Angiogenic Therapies
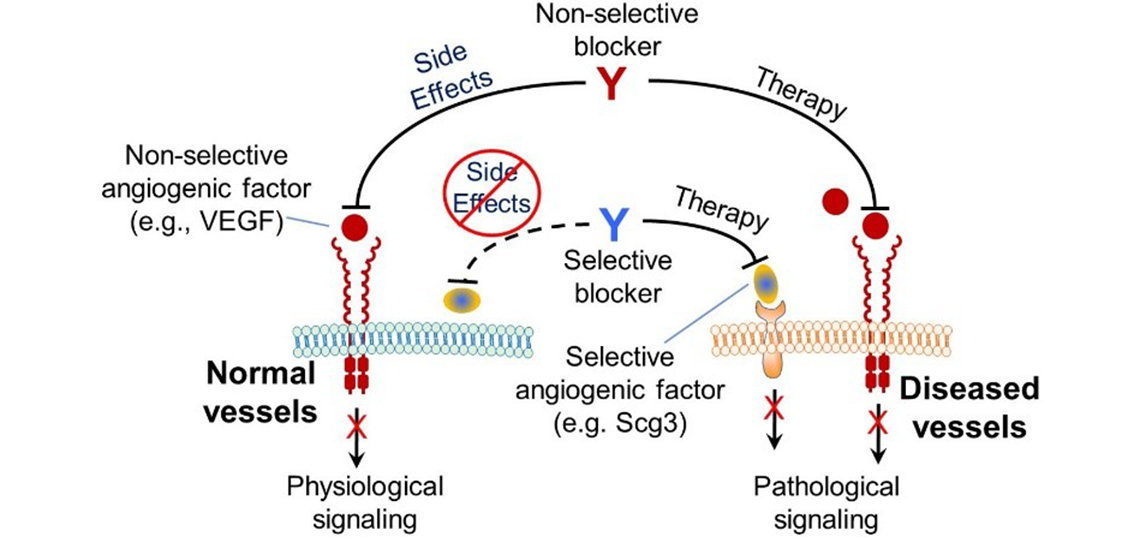
Disease-targeting anti-angiogenic therapy. Scg3 preferentially stimulates angiogenesis in diseased versus healthy vessels, whereas conventional angiogenic factors, such as VEGF, regulates both diseased and healthy vasculatures. Anti-Scg3 antibody selectively blocks Scg3 function in diseased vessels with minimal side effects on healthy vasculatures. In contrast, anti-VEGF drugs simultaneously exert both therapeutic and untoward effects on diseased and healthy vessels.
Secretogranin III (Scg3) was discovered as a disease-selective angiogenic factor by our comparative ligandomics in an animal model. Our studies revealed that Scg3 selectively binds to and induces angiogenesis of diseased but not healthy vessels, whereas conventional angiogenic factor, such as VEGF, binds to and simulates angiogenesis of both diseased and healthy vasculatures.
We generated Scg3-neutrizing antibodies and demonstrated their high efficacy to alleviate vascular diseases, such as diabetic retinopathy, neovascular age-related macular degeneration and retinopathy of prematurity, in animal models.
We are developing anti-Scg3 antibody not just as another novel anti-angiogenic drug but a founding member of a new class of next-generation disease-targeting angiogenesis blockers. The advantage of this targeted anti-angiogenic therapy is minimal side effects on normal blood vessels and cells with wide therapeutic windows. We are currently developing anti-Scg3 antibody toward clinical trials.
Other disease-selective targets discovered by our comparative ligandomics are in various stages for drug development, ranging from target validation to neutralizing antibody generation and characterization.
Our drug development program has led to two issued and pending patents for anti-Scg3 therapy. A startup biopharmaceutical company has been funded by two NIH business grants to commercialize the therapy.








