Estrogen Receptors in Breast Cancer Progression
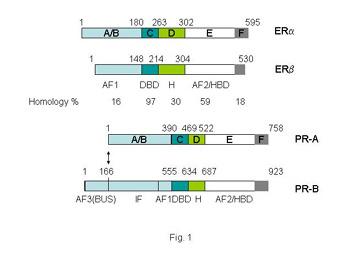
Many human breast cancers are dependent on the steroid hormones, estrogen and progesterone for their growth, and their effects are mediated through the estrogen receptors (ERs α and β) and progesterone receptors (PRs A and B), PDF. The steroid receptors have a complex domain structure as shown in Figure 1.
Treatment
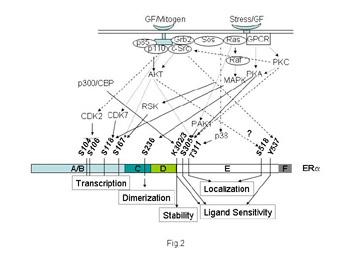
Treatment directed at reducing the mitogenic effects of estrogen through blockade of the ERs was the first (Beatson 1896), and remains the most frequently prescribed form of breast cancer therapy. But while this strategy is initially useful in many breast cancer patients, acquired resistance eventually develops with the appearance of metastatic lesions in patients. An emerging hypothesis is that the ERs still remain central to the problem of resistance, due to their molecular crosstalk with growth factors, or possibly other intracellular signaling molecules. Our goal in the lab is to dissect the role of the ER signaling network for assessing clinical outcome and selecting appropriate therapies for ER-positive breast cancer.
A Hypersensitive ER Alpha Mutant in Breast Cancer Progression
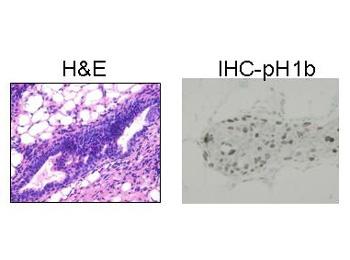
Our laboratory discovered a somatic mutation in ER alpha, replacing lysine 303 with arginine (K303R ER alpha), in about a third of clinical premalignant breast ductal hyperplasias (PDF). Transgenic mice expressing the mutation in the mammary gland likewise develop highly proliferative ductal hyperplasias (H&E and immunohistochemistry with phosphorylated histone H1B of a hyperplasia arising in mutant ER transgenics are shown below). The mutant ER is hypersensitive to estrogen, suggesting that it might drive inappropriate growth and therefore propel these hyperplasias toward malignancy.
We are studying the molecular basis for the hypersensitive phenotype of the ER mutation in a funded NIH RO1 project. Our goals are to:
1. Determine whether the presence of the K303R ER mutant is prognostic for recurrence of primary breast cancer.
2. Determine how the K303R ER mutant is associated with the progression of early breast lesions toward invasive breast cancer.
3. Define the molecular mechanisms underlying the hypersensitive ER mutant phenotype by studying the association of mutant vs. wild-type ER alpha with critical co-activators and co-repressors, the dynamics of mutant vs. wild-type ER/co-regulator complexes on specific promoter DNAs, using ChIP and co-immunoprecipitation assays, ubiquitin-mediated degradation, and protein acetylation PDF.
4. Examine tumorigenesis in xenograft athymic mice models.
Role of the Estrogen Receptor Alpha Mutation in Breast Cancer Metastasis
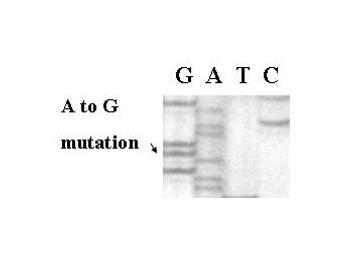
Dideoxy sequencing of human specimens has revealed that the K303R ER mutation is present in invasive breast cancers (see mutation in Figure 4).
We hypothesize that activation of ER alpha by mutation at the K303 site is a “permissive” event occurring early during breast cancer evolution, and that its hypersensitive phenotype provides a proliferative advantage which could favor tumor metastasis. As a funded project within the Baylor Breast SPORE link to funding, we are studying:
Metastatic Behavior

1. Whether the K303R ER alpha mutation influences the metastatic behavior of breast cancer cells. We are sequencing for the mutation in metastases removed from breast cancer patients, and examining the mutant receptor’s influence on metastatic behavior in xenograft models. Lung metastases that arose in athymic mice with ER alpha-expressing breast cancer cells grown as xenografts is shown in Figure 5.
Outcome of Hormonal Therapies
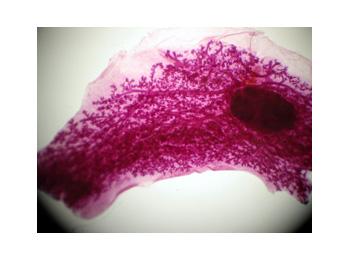
2. Whether the mutation alters outcome of hormonal therapies, in specimens from a clinical trial, and in a bitransgenic mouse models crossing the mutant with c-myc and HER-2 oncogene transgenics. A whole mount of a mammary gland from a K303R mutant transgenic animal, and a pituitary isograft are shown to the right.
3. And finally, how the mutation might be exploited as a target for new therapeutic strategies.
Prognosis of Breast Cancer
Selecting breast cancer patients with micrometastases at diagnosis is crucial for deciding who should and who should not receive toxic and expensive adjuvant chemotherapy to eradicate these metastatic cells. Axillary nodal status, the best marker available, still misclassifies about one-fourth of patients. Dr. Fuqua and other investigators in the Breast Center are utilizing powerful molecular techniques to identify new prognostic markers.
Integrating RNA Expression and CGH Profiles to Predict Long-term Breast Cancer Progression
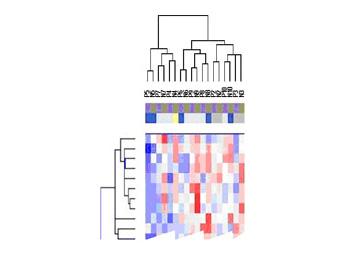
Breast cancer is characterized by a long natural history, and a very heterogeneous clinical course. Because of this unpredictability of breast cancer, the majority of patients are treated for their disease, because we have few biomarkers that reliably predict metastasis. As part of our funded Program Project grant, we are using microarray expression analysis of tumor samples from node-negative patients who received no adjuvant therapy, and with very long clinical follow-up (>12 years), in order to generate clinically useful profiles that more accurately predict long-term outcome (see Figure 7.)
These studies will identify genes and pathways important in the metastatic process of breast cancer patients for future biological studies. We will develop metastatic breast cancer mouse model systems to then study the identified genes.
Dissection of the Role of Src Family Members in Breast Cancer Metastatic Behavior
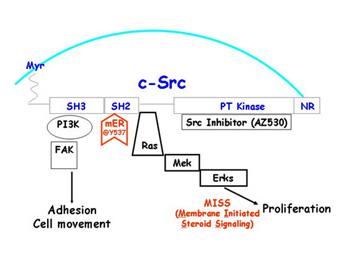
We have developed ER alpha - positive breast cancer models to dissect the role of the Src tyrosine kinase in invasion and metastasis of breast cancer cells. We have data suggesting that Src signaling may play a role in the hormone-dependent metastatic behavior of breast cancer cells.
We are funded by Astrazeneca to study Src signaling in our ER alpha-positive breast cancer cells. We are evaluating the role of different Src kinase members as modulators of activity both up-stream and down-stream of ER signaling. We will also examine the effects of Src inhibitors on the transcriptional activity, and the effects of altered Src signaling on the invasive behavior of breast cancer cell lines stably expressing both the K303R ER alpha mutant and wild-type ER.
Tamoxifen Resistance
Tamoxifen (Tam) antagonizes the growth-promoting effects of ERs alpha and beta. However, not only does resistance to Tam develop, but there are toxicity problems with this drug. We propose to attack the problem of resistance by identifying novel genes which cause or impact on resistance, so that future specific therapies can be developed to these genes, or so these genes can be used as biomarkers to avoid hormonal therapies if the likelihood of developing resistance exists.
Tamoxifen Resistance
Tamoxifen (Tam) antagonizes the growth-promoting effects of ERs alpha and beta. However, not only does resistance to Tam develop, but there are toxicity problems with this drug. We propose to attack the problem of resistance by identifying novel genes which cause or impact on resistance, so that future specific therapies can be developed to these genes, or so these genes can be used as biomarkers to avoid hormonal therapies if the likelihood of developing resistance exists.
Cytoskeleton Organization and Tamoxifen Resistance
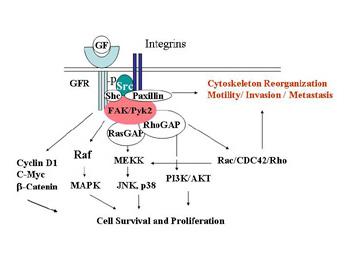
An understudied avenue of research in antiestrogen resistance is the involvement of the actin cytoskeleton, and its reorganization with hormonal therapies. The consequences of actin reorganization are the tumor-associated processes of motility, invasion, and metastasis.
We hypothesize that growth factor-mediated second messenger signaling and ER alpha is tightly involved in the reorganization of the actin cytoskeleton of the cells, thereby influencing cellular response to antiestrogens. In a recently funded Concept Award from the Department of Defense, we are examining whether estrogen and/or growth factors alter the expression of molecules involved in actin filament organization, and whether estrogen and/or growth factors alter the structural localization of actin binding proteins (ABPs). We plan to dissect the signaling molecules involved using specific inhibitors of second messengers, and to utilize siRNA methodologies to define the contribution of individual ABPs to antiestrogen resistance. This research could directly impact on our ability to predict those women who will develop resistance, and to develop future therapies to reverse resistance.
Protein Phosphatases and Tamoxifen Resistance
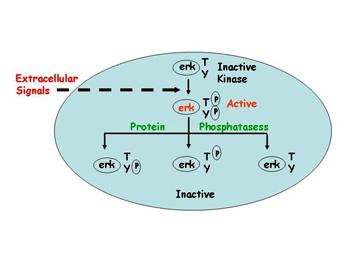
Using microarray expression analysis, we have discovered that certain protein phosphatases are elevated in women whose tumors recurred and metastasized during the course of their treatment with Tam. We have begun to determine if these phosphatases indeed cause resistance in different model systems, and we will then dissect exactly how they do so. These phosphatases dephosphorylate important intracellular signaling molecules which have already been shown to be important in breast cancer progression. But almost nothing is known about the breast cancer role of protein phosphatases and whether they can influence ER signaling and the development of resistance in patients.
We plan to determine whether overexpression of this class of proteins confer resistance to Tam in ER-positive breast cancer cells using genetic engineering, and then investigate their growth in a preclinical xenograft models of human breast cancer. We will also use inhibitors of these signaling pathways, and siRNA knock-down experiments to reduce the levels of potential ER accessory proteins or signaling molecules.








