As a result of our work with T1D in the TEDDY and nPOD projects, it has become clear that significant molecular biological work must be done to determine mechanisms that can enable enteroviruses to cause or trigger T1D. Disparate results have been reported concerning virus infection in pancreatic beta cells and also in various immune cells. We are investigating the molecular biology of Coxsackie B virus infection in three human beta cell lines and human white blood cells, particularly plasmacytoid dendritic cells.
Our hypothesis is that one or more of these cells cause viruses to enter into defective RNA replication mechanisms that result in deletion of up to 50 nucleotides from the 5’ end of the genome. This enables the virus to switch from cytolytic mode for replication to long term chronic, non-cytolytic infection. We are endeavoring to discover underlying mechanisms that drive this process and link them back to specific cellular gene expression patterns and host factors.
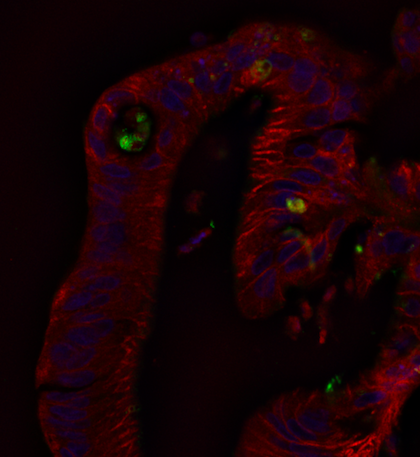
We are also investigating enterovirus replication in human enteroid models of gut mucosa. Despite all that is known about enteroviruses, a good deal of mystery surrounds the sites of virus replication in the human alimentary tract. Further, the host restrictions that govern cell tropism for the virus are very unclear and likely extend far beyond the presence or absence of suitable receptors for the virus.
We are collaborating with Dr. Mary Estes in the MCVM department to investigate virus replication in a series of enteroids derived from human duodenal, illeal, jujeunal sections as well as from stem cells. Since the gut serves as the portal for viral penetration to the bloodstream, it is important to learn the role of intestinal cells in promoting or restricting virus access to secondary sites in the body that lead to diseases such as myocarditis, potentially type 1 diabetes and neurological outcomes such as meningitis and paralysis.








