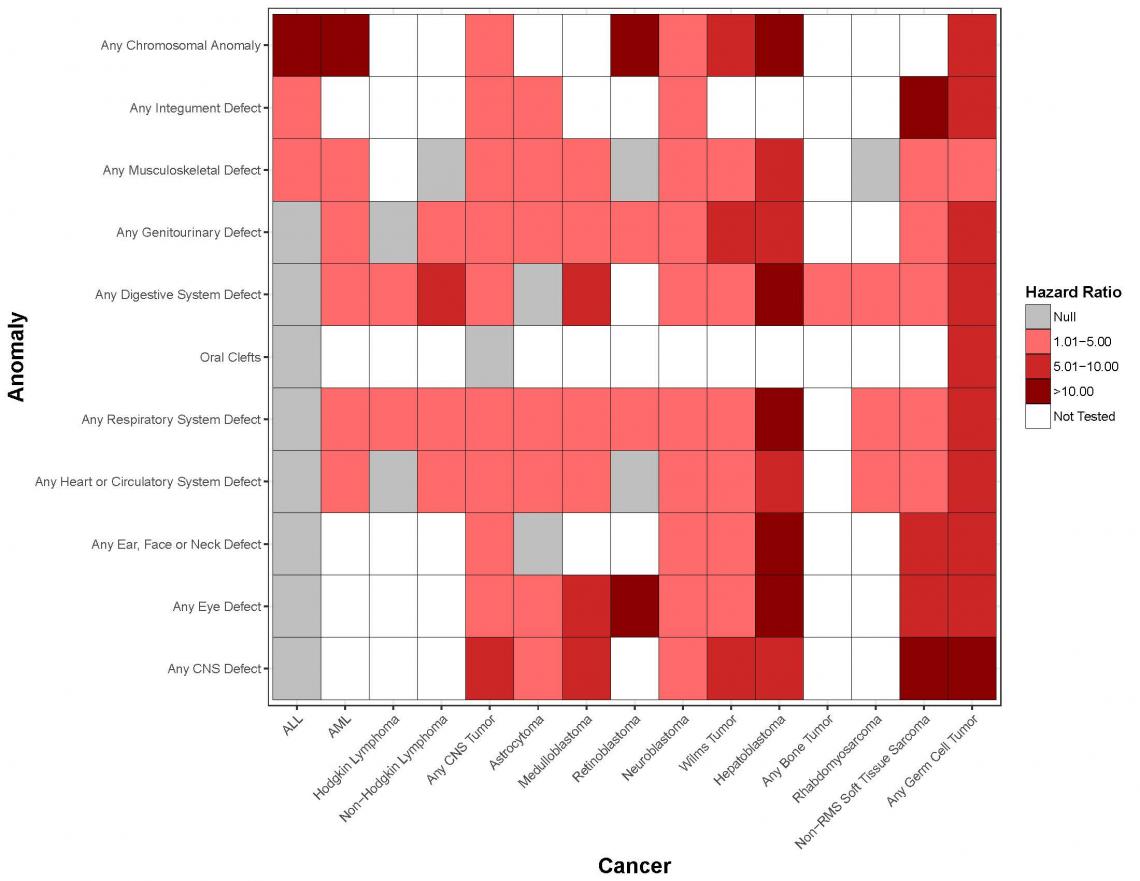
Genetic Overlap Between Anomalies and Cancer in Kids (GOBACK)
Globally, more than 250,000 children are diagnosed with cancer every year. In the United States, cancer remains the leading cause of death by disease in those under 20 years of age. One of the strongest risk factors for cancer in children and adolescents is being born with a congenital anomaly-this is true both for chromosomal abnormalities (e.g., Down syndrome) and non-chromosomal birth defects (e.g., non-syndromic congenital heart defects), as recently validated in our registry linkage study of over 10 million live births. As an estimated 8 million children worldwide are born with a congenital anomaly per year (6 percent of all births), the public health implications of identifying why these children develop cancer are substantial. In the GOBACK Study, we will address current gaps that limit translational impact by:
- Identifying and confirming recurrent congenital anomaly patterns that predispose children to cancer.
- Characterizing the molecular etiologies underlying these observed associations, which has the potential to identify new and important cancer predisposition syndromes and the relevant genes among children with congenital anomalies.
This study has been funded by Alex’s Lemonade Stand Foundation, the Cancer Prevention and Research Institute of Texas, and the Department of Defense.
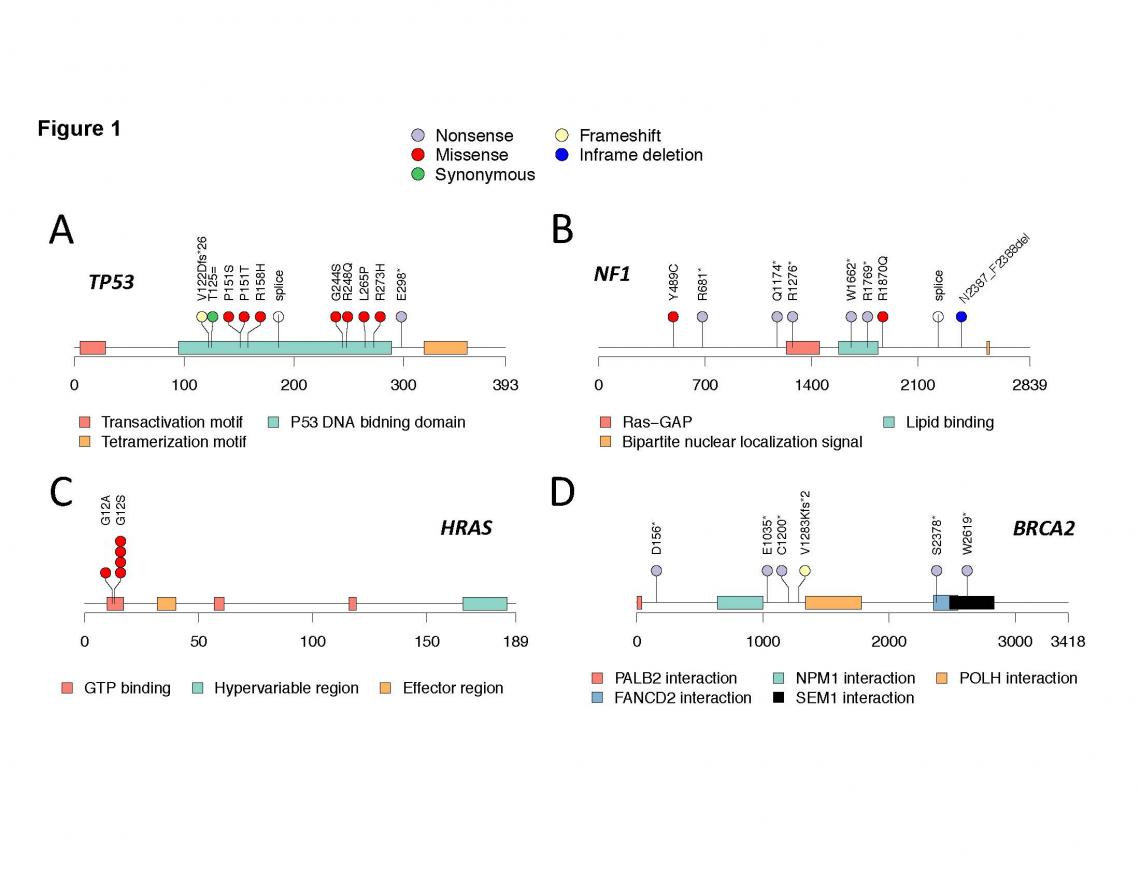
Genetics of Embryonal and Alveolar Rhabdomyosarcoma Study (GEARS)
Rhabdomyosarcoma (RMS) is a highly malignant tumor believed to arise from developing skeletal muscle cells (myoblasts). Relative to other childhood cancers, the prognosis for many children with RMS remains poor. In particular, for those children with high- or intermediate-risk disease, use of maximally intensive therapy and application of new agents since the 1970s has led to only modest improvements in 5-year survival rates, which is currently only 43 percent to 67 percent. Through collaborations in the Children’s Oncology Group, as well as funding through the Cancer Prevention and Research Institute of Texas and the National Institutes of Health, we have developed GEARS to comprehensively characterize the role of inherited genetics on susceptibility to this highly fatal tumor.
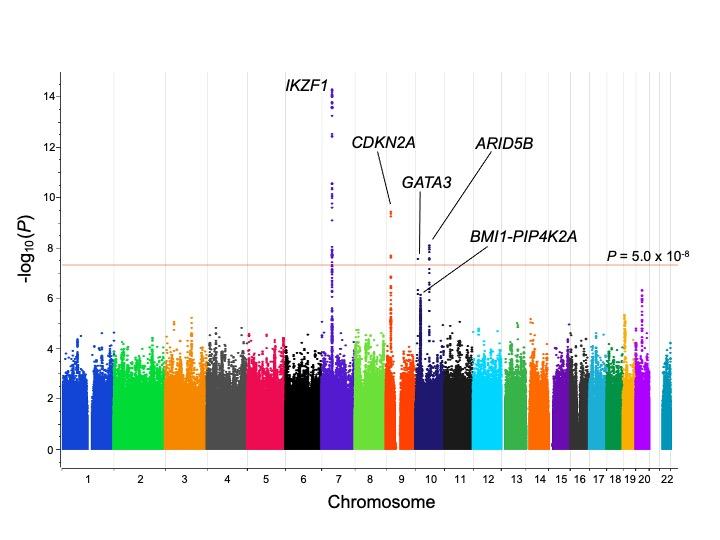
Down Syndrome Phenotyping of Acute Leukemia (DS-PAL) Study
Children with Down syndrome (DS), which occurs due to trisomy 21, have a 20-fold increased risk of acute lymphoblastic leukemia (ALL), but the basis for the increased risk of leukemia remains unclear, including the potential interplay between other DS phenotypic features and ALL susceptibility. This study will build upon our previous genome-wide association study, as well as our ongoing genomic profiling and phenotyping efforts to:
- Perform a comprehensive analysis of heritable variation associated with risk of ALL in children with DS, with a focus on structural, rare and chromosome 21 variants.
- Conduct deep phenotyping of children with DS-ALL to identify the impact of DS-related phenotypes on leukemia susceptibility and outcomes.
Findings from this study may lead to improved genetic testing and counseling strategies for children with DS, and insights into genes driving DS-ALL may guide development of targeted therapies to improve outcomes in this vulnerable and high-risk patient population. This work is currently funded by the National Cancer Institute.
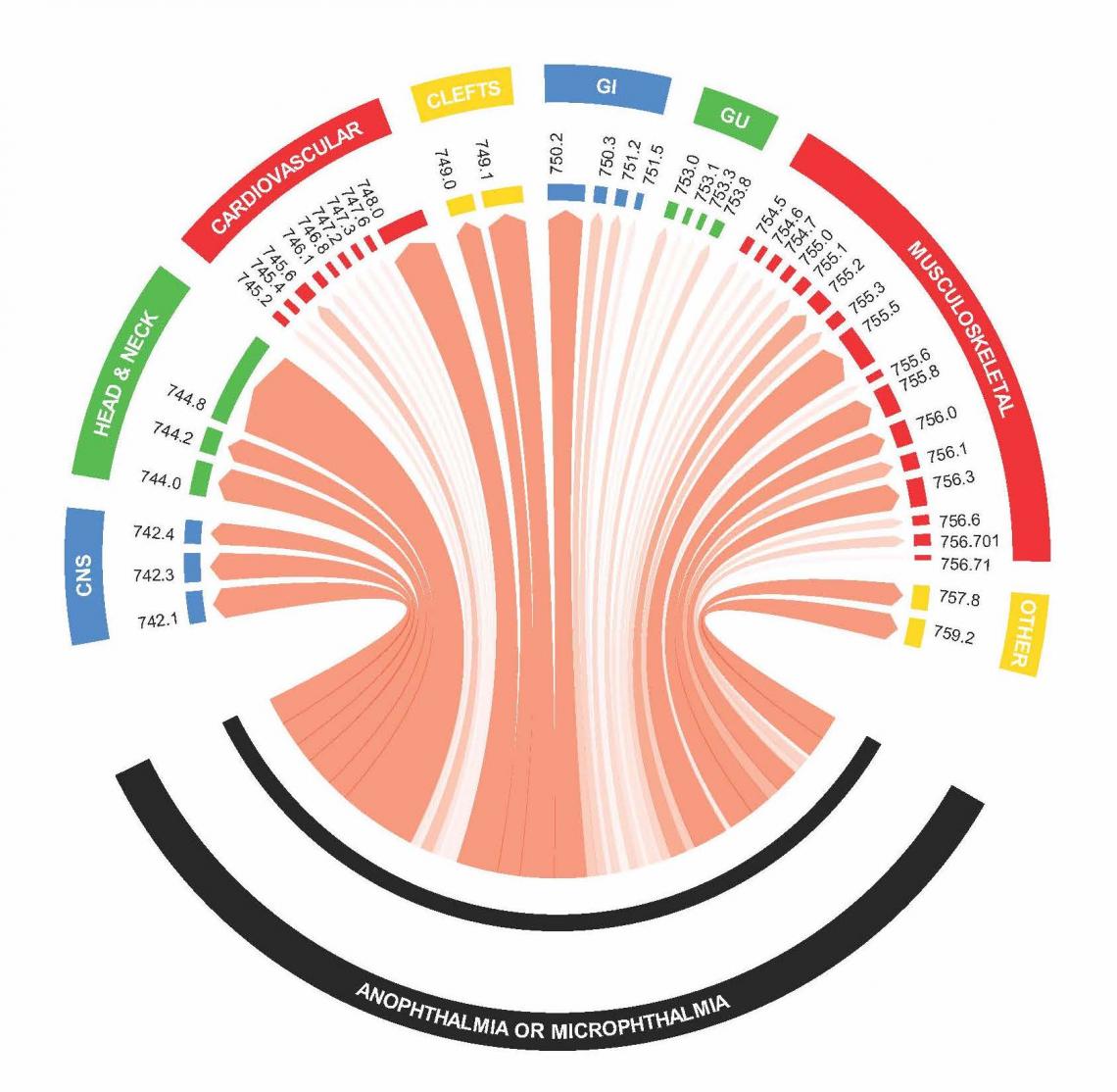
Genetics and Epidemiology of Multiple Malformation Syndromes (GEMMS) Study
Over 50 percent of children with multiple malformations seen in medical genetics clinics for a suspected genetic syndrome never receive a diagnosis, which leaves unanswered questions about prognosis and medical/reproductive planning. Our study is relevant to public health because we will identify new multiple malformation syndromes (MMS), characterize these MMS in a clinical population, and explore the genetic underpinnings of these syndromes. These results would lead to:
- Expectations for disease progression and improved medical management and reproductive planning.
- The foundation needed for the development of therapies that could improve the health of affected patients and/or prevent the occurrence of these MMS.
This work has been funded by the National Institute of Child Health and Human Development.
Reducing Ethnic Disparities in Acute Leukemia (REDIAL) Consortium
Despite improved outcomes for pediatric leukemia, ethnic disparities persist. Notably, Hispanics suffer both worse survival and increased treatment-related toxicities. There is a critical need to discover biomarkers for adverse outcomes in at-risk populations. The REDIAL Consortium will expand and enhance our recently created population-based research network in an area characterized by a large and growing Hispanic population to generate prospective data and samples on about 1,000 children. We hypothesize that clinical, genomic, and biological information will enable the identification of leukemia patients at increased risk for chemoresistance, relapse, and toxicity, thereby guiding treatment decision-making and improving prognosis in at-risk populations. Our ultimate goal is to identify children at the greatest risk of toxicities and relapse.
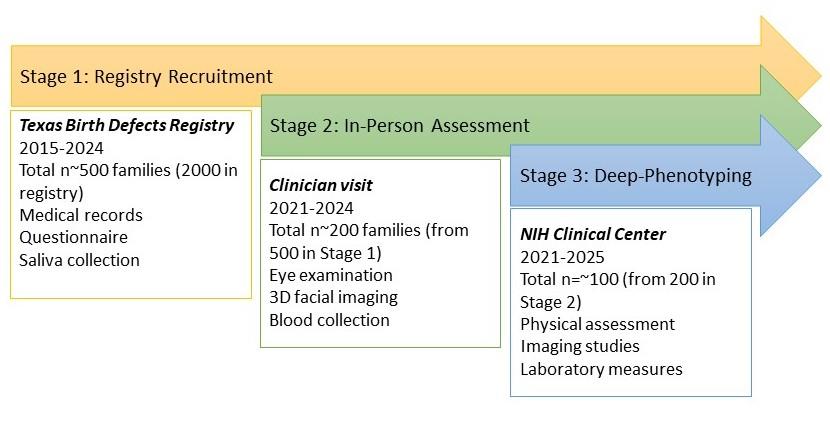
Microphthalmia, Anophthalmia, and Coloboma Genetic Epidemiology in Children (MAGIC) Study
Among the more common visually threatening congenital eye defects are anophthalmia (total absence of the globe); microphthalmia (anomalously small eye in the orbit); and coloboma (failure of the closure of the fetal fissure) – collectively referred to as MAC. There have been few if any population-based studies to:
- Describe the proportion of cases due to pathogenic variants in known MAC-related genes.
- Systematically characterize co-occurring phenotypes in these individuals (herein termed “deep phenotyping”), which could provide insights into the underlying etiologies of these conditions and inform precision medicine efforts.
Therefore, objectives of the current study are to
- Better define the MAC phenotype.
- Characterize the role of known and potentially novel pathogenic genetic variants that confer MAC susceptibility.
As part of this, we will utilize the resources of the National Institutes of Health (NIH) Clinical Center to comprehensively phenotype cases with MAC, as well as the National Eye Institute (NEI) Ophthalmic Genomics Laboratory and Human Genome Sequencing Center at Baylor College of Medicine to identify genetic variants underlying MAC phenotypes.
For study related questions contact the EpiCenter study team at 713-798-2920 or epicenter@bcm.edu.








