Identifying the origins of neck brown adipose tissues
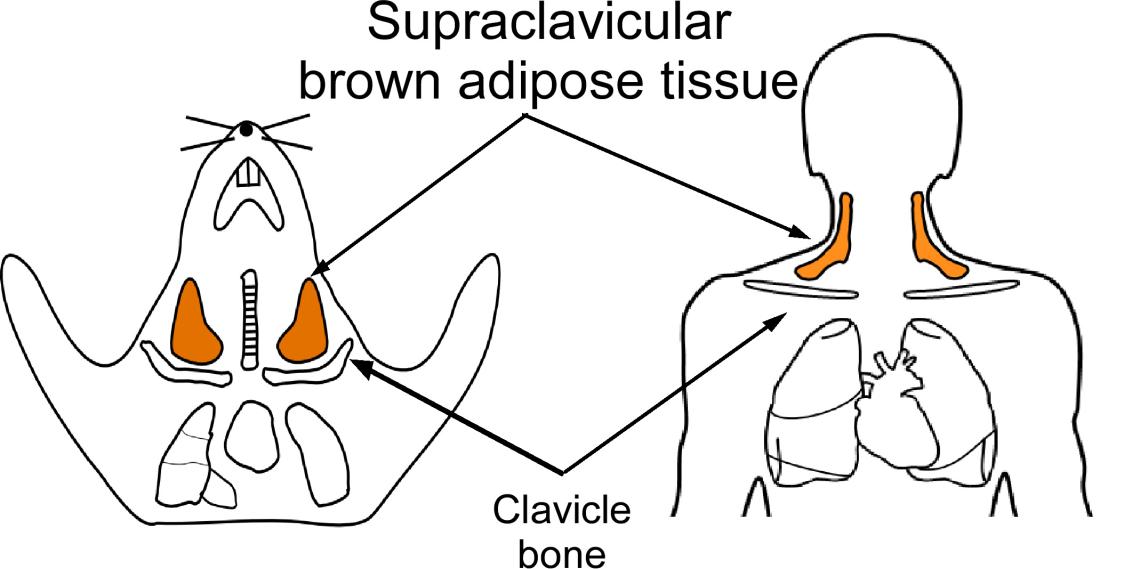
Brown adipose tissue (BAT) can utilize nutrients to generate heat and, thus, can maintain body temperature and increase energy expenditure. Because of this unique feature, BAT has been recognized as a potential therapeutic target for treating obesity. Healthy adult humans possess brown adipose tissue in the neck, with the most permanent depot located above the clavicle bone, named the supraclavicular brown adipose tissues (scBAT). To understand human scBAT development and function, we identified a previously unknown brown adipose tissue depot in the mouse neck, in the same area as human scBAT. We named the depot, mouse scBAT (Shi et al., Cells. 2021; Mo et al., JCI Insight. 2017). To probe the origin of scBAT, we applied genetic lineage tracing techniques and have identified multiple developmental lineages that can give rise to scBAT. Currently, we are working on determining the developmental trajectories of these progenitor cells and investigating the functional contribution of scBAT adipocytes to thermogenesis and energy expenditures.
Identifying beige adipocyte progenitor cells and investigating how environmental temperature, obesity, and aging impact their ability to generate beige adipocytes in mice
The beige adipocyte, a new type of thermogenic adipocyte, exists in white adipose tissues. Like brown adipocytes, beige adipocytes can convert chemical energy to heat and thus, possess the ability to potentially regulate energy expenditure and combat obesity. To isolate lineage-specific beige adipocytes from heterogenous adipocyte populations in white adipose tissue, we developed a novel intersectional lineage tracing system to trace lineage-marked beige adipocytes in mice. Using this system, we plan to trace, and isolate lineage-marked beige adipocytes and systematically analyze the cellular and molecular characteristics of these adipocytes in mice exposed to different environmental stimulants, cold and diet.
Determine the maternal effects on offspring adipocyte progenitor cell development
Maternal high-fat diet has detrimental effects on offspring health, leading to the increase in the chance for offspring to develop chronic metabolic-related diseases, such as obesity and type II diabetes. Adipose tissue is one of the tissue types that impacted by maternal high-fat diet. However, the underlying mechanisms that mediate the maternal effect on offspring adipose tissue development have not been well-studied. We are generating a novel lineage tracing system to genetically mark the adipocyte progenitor cells in offspring during development. In doing so, we will be able to trace the development of these adipocyte progenitor cells under the influence of maternal high-fat diet.








