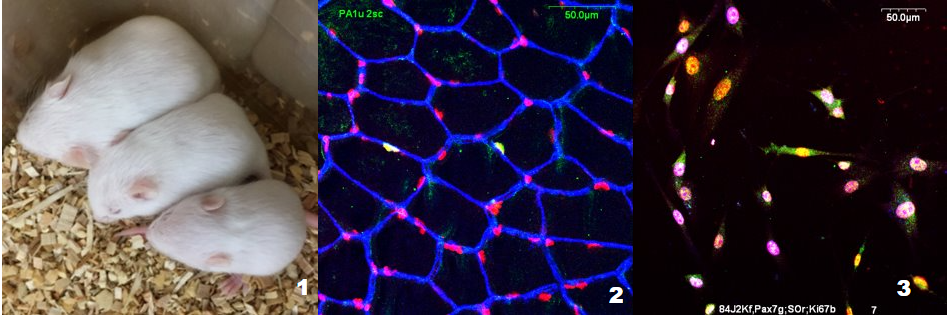
Regulation of skeletal muscle satellite cell function by protein intake during the postnatal period
When animals are undernourished in the newborn period, skeletal muscle growth is significantly compromised. Nutritional rehabilitation after weaning does not recover muscle mass. We have been undertaking a series of experiments to understand why this is so. Such information is necessary to design interventions that have a chance of successfully restoring muscle mass. Our goals are: (a) to assess the contribution of programmed alterations in protein turnover and satellite cell proliferation to the impaired growth; (b) to understand the mechanisms that result in these defects in cellular function and; (c) to identify the specific dietary nutrient deficit(s) that are responsible for the occurrence of the impairments.
These studies are primarily performed in mice in which we cross-foster pups at various developmental ages between dams fed a control or a reduced protein diet (to reduce milk production). Alternatively, we alter litter size to decrease or increase the amount of milk available to individual pups. We use: tracers to measure protein turnover and satellite cell proliferation in vivo; a variety of biochemical and molecular techniques to assess the activity of cellular signaling pathways, gene expression, enzyme activities, protein expression and hormone and metabolite levels; histological methods to characterize muscle phenotype; isolation of muscle progenitor cells for in vitro studies to identify inherent functional deficits resulting from the in vivo nutritional experiences of the organism.
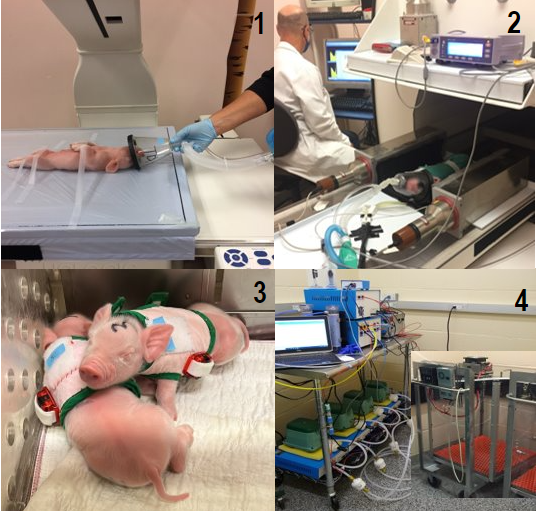
Evaluation of chronic dietary supplementation with leucine or β-hydroxy β-methyl butyrate on muscle growth and body composition
Short-term supplementation of diets of newborn animals with leucine or its metabolite, β-hydroxy-β-methylbutyrate (HMB), stimulates muscle protein synthesis and satellite cell proliferation. We are conducting studies to determine if these anabolic effects are sustained and promote greater muscle protein accretion when the supplements are fed long-term. We are also assessing the effect of differences in lean mass on the partitioning of dietary energy intake to better understand its influence on energy balance and fat deposition in the neonate.
These studies are performed in full-term newborn pigs that are fed artificial formulas that mimic sow’s milk. We use many of the same techniques listed for our mouse studies. In addition, we perform longitudinal measurements of: total body potassium and dual x-ray absorptiometry to quantify changes in fat, lean, bone, and cellular masses; activity by accelerometry; energy intake and whole body energy expenditure by indirect calorimetry to determine energy balance.
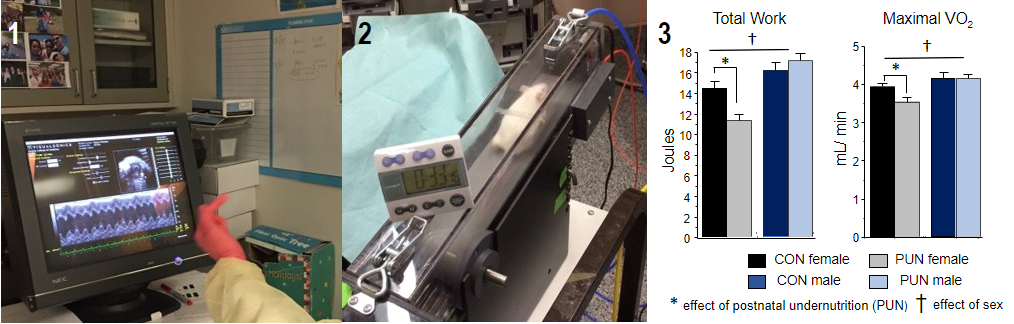
Interventions to mitigate long-term cardiac dysfunction resulting from early life undernutrition
Impaired growth during fetal life can reprogram heart development and increase the risk for long-term cardiovascular dysfunction. We found that, compared to males, young adult females that had been undernourished in early postnatal life and then nutritionally rehabilitated had disproportionately smaller and dysfunctional hearts with compromised maximal exercise capacity. With progressive aging, many of the functional risks that develop are similar to those that predispose to heart failure with preserved ejection fraction, a condition that is most prevalent in women. Thus, there appears to be a developmental window during which the heart is undergoing terminal maturation when it is vulnerable to reprogramming by inadequacies in nutrient intake. Our long-term goal is to reveal the mechanistic basis for the cardiac anomalies that develop as nutritionally compromised infants age and to identify preventive strategies to mitigate disease progression.
These studies are primarily performed in mice using the cross-fostering and protein-restricted diet model to induce undernutrition and rehabilitation. In vivo assessment of cardiac function and size are performed in collaboration with the DeBakey Heart Center Murine Non-Invasive Cardiovascular Function Laboratory. Exercise testing (VO2 peak, endurance, strength, voluntary activity), body composition, and measurements of energy expenditure and substrate utilization are performed in the Mouse Metabolic Research Unit at the CNRC.
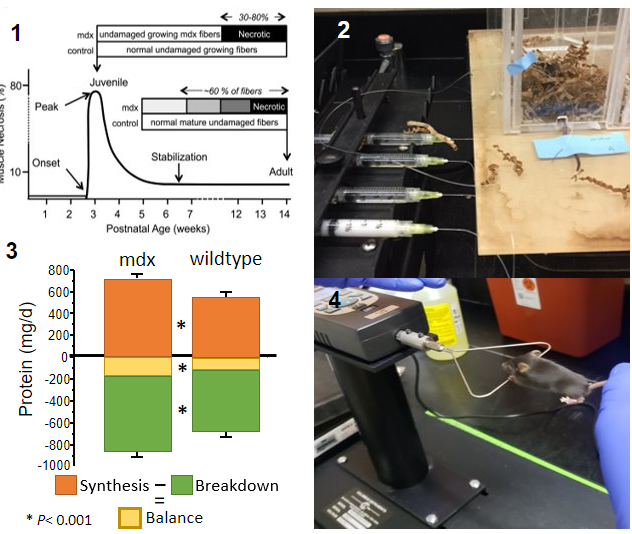
Dietary protein requirements and citrulline supplementation to support growing dystrophic muscle
Little is known about protein requirements in patients with neuromuscular diseases. Current recommendations are that protein intake should meet the normal recommendations for age as there is little evidence to suggest otherwise. Our research on the regulation of muscle and protein metabolism in highly anabolic conditions would indicate that this may not be so. Additionally, the genetic defects are likely to incur alterations in the metabolism of specific amino acids and, hence, their nutritional requirements. We are using a mouse model of Duchenne muscular dystrophy to define the contribution of skeletal muscle protein turnover to whole body protein turnover, how this affects dietary protein requirement, and the impact of variations in the dietary protein:energy ratio on the severity of muscle pathology. Citrulline is a precursor for arginine that serves as a substrate for the synthesis of many critical molecules, in addition to protein. During growth, and other anabolic or inflammatory conditions arginine needs are increased. We are conducting studies in the dystrophic mouse model to assess if supplementation with citrulline confers any benefits in mitigating the progression of the disease.
Our studies are conducted in the mdx mouse model of muscular dystrophy. They are performed when the disease process is most severe, that is from the preweaning period, when muscle damage is first observed, through to adolescence. We use stable isotope amino acid tracers to assess protein turnover in vivo; a wide variety of histological, molecular, and biochemical assays to assess disease severity; an array of in vivo measurements to assess muscle function; measurements of body composition, food intake, and indirect calorimetry to evaluate the efficiency of dietary protein and energy utilization.








