Paligenosis and Recruitment of Progenitor Cells
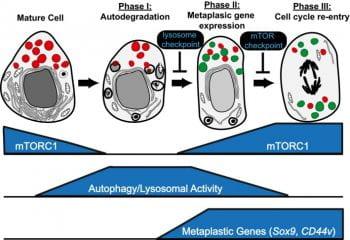
Paligenosis is how differentiated cells return to the cell cycle. It is a cellular program that is evolutionarily conserved across organs and species, similar to how apoptosis is an evolutionarily conserved program to execute cell death. Paligenosis begins with massive upregulation of autophagy and lysosomes as the cell begins to recycle organelles to convert from a mature, secretory state to a trimmer, progenitor-like state. After the autophagy stage, cells undergoing paligenosis upregulate expression of proteins associated with the embryonic and wound healing state: SOX9, nuclear YAP1, Mucins. They then decide whether to undergo cell division or not. Paligenosis seems to be required for metaplasia, a precancerous lesion that occurs during chronic injury and inflammation. Projects in the lab are to dissect the cell biological and molecular regulation of paligenosis, which involves how paligenosis-specific proteins (eg, ATF3, DDIT4, IFRD1) interact with regulators of cell energetics and injury response (eg, mTORC1, p53). These basic investigations inform projects that are more translational or clinically based as we work with organoids derived from actual patients with metaplasia and cancer to determine how paligenosis regulates cancer risk and progression.
Stem Cells and Metabolism Regulating Differentiation
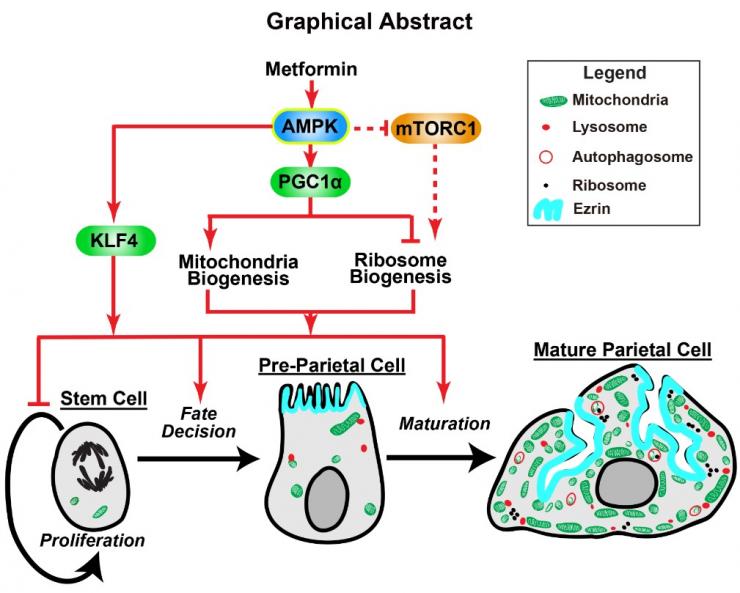
In tissues with constitutively dividing undifferentiated stem cells (like the epithelium of the GI tract), the stem and early progenitors themselves can respond to injury to help regenerate tissue after damage. For example, chronic inflammation in the stomach – which puts people at risk for gastric cancer – causes loss of acid-secreting parietal cells. This parietal cell atrophy somehow increases risk for cancer. It also can lead to an attempt by stem and progenitor cells to regenerate the parietal cells. Projects in the lab study how stem cells, in the normal state and in injury, decide what cell type to become (eg parietal cells). For example, we have shown stimulating energetic pathways organized by the cellular energetic hub protein AMPK induces stem cells to become parietal cells by mechanisms involving PGC1α. The drug metformin stimulates AMPK-PGC1α and thereby causes more stem cells to choose to become parietal cells. We are studying the cellular (eg organelles and trafficking) and molecular (eg proteins interacting with AMPK) aspects of how metabolism affects stem cell differentiation with the thought that restoring normal differentiation could help decrease risk for cancer.
Therapy for Metaplasia and Cancer
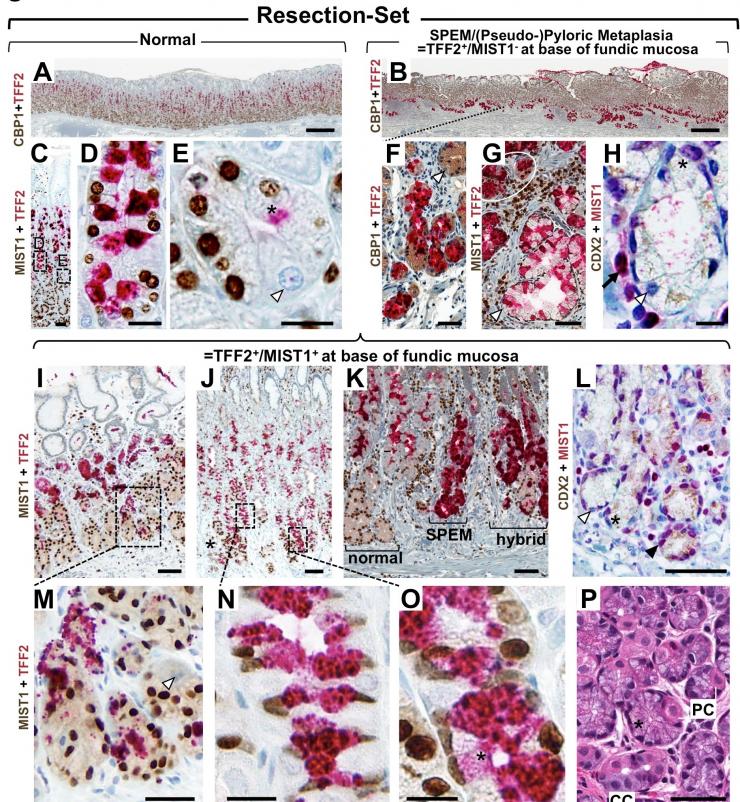
The lab has projects of a more translational and clinical nature involving large databases of human tissue showing precancerous lesions (eg metaplasias) in several tissue but in particular stomach, esophagus, and pancreas. The lab also has collected and grows organoids from normal, metaplastic, and cancerous human organs. We characterize how chemotherapy drugs affect growth of cancer patient organoids with the hope to tailor therapy. And we use multi-omics approaches (sc-RNA-Seq, Spatial transcriptomics) to determine how metaplasia occurs and how it might progress to dysplasia and cancer.








