In Eukarya, RNA Polymerase II (Pol II) transcription is a highly regulated process, which is responsible for the transcription of all protein encoding genes. While the catalytic core of the Pol II transcription machinery is the same for both basal (activator-independent) and activated transcription as observed in vitro and in vivo, respectively, transcription activation requires additional coactivators that function either by modifying chromatin or more directly by facilitating the assembly and function of the preinitiation complex (PIC).
It is now widely accepted that binding of the TATA-box binding protein (TBP) to the TATA-box causes a bending and severe deformation of the DNA. TBP binding to the TATA-box nucleates PIC formation either through a stepwise assembly of other basal transcription factors or through recruitment of a pre-assembled Pol II holoenzyme. Intriguingly, biochemical studies showed that the core domain of TBP (TBPc) recognizes the TATA-box in both orientations with only a 60:40 preference for the correct orientation. This is supported by numerous crystal structures of a TBPc-DNA complex that revealed pseudo two-fold rotational symmetry of the TBP-DNA interface. Hence, it is unclear what determines the orientation of PIC assembly and, thus, the direction of transcription. To provide the structural basis for the polarity of transcription, we determined the 2.65-Å resolution crystal structure of a human ternary TBP-TFIIB-DNA complex, consisting of the core domains of human TBP (TBPc) and human TFIIB (TFIIBc) bound to an idealized and extended adenovirus major late promoter (PDB: 1C9B). Most remarkable, our structure reveals five ternary complexes in the crystallographic asymmetric unit, which are linked through a single base-pair overhang at the 5'-end of the oligonucleotide used for crystallization (Figure 1).
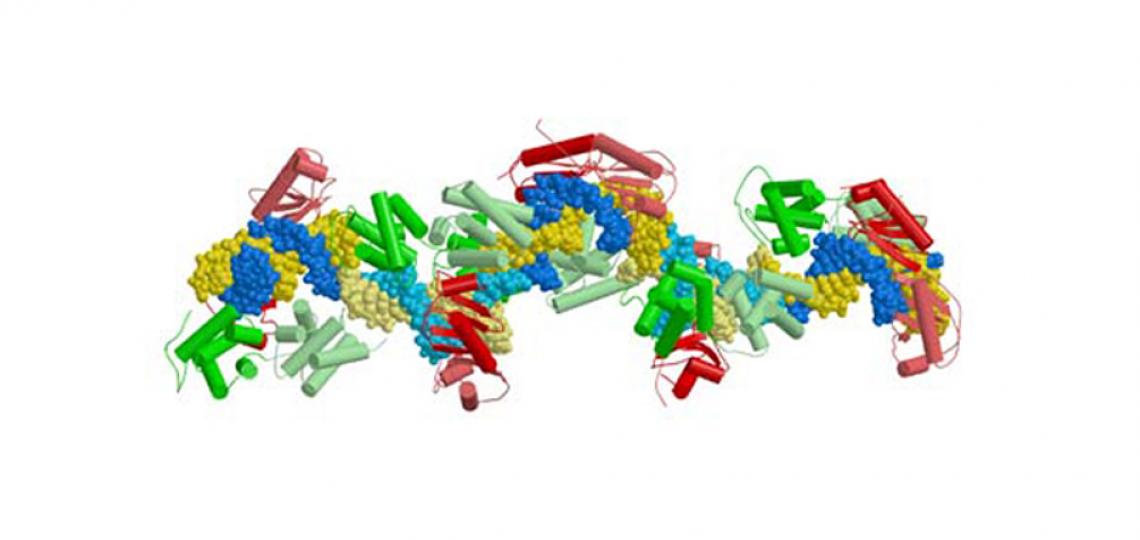
Figure 1: Architecture of a human ternary TFIIBc-TBPc complex bound to a 17 base pair long segment of the adenovirus major late promoter (Tsai and Sigler, EMBO J. 2003)
Figure 1: Architecture of a human ternary TFIIBc-TBPc complex bound to a 17 base pair long segment of the adenovirus major late promoter. Human TBPc is colored in red and human TFIIBc in green. The figure depicts the content of one asymmetric unit. There are five ternary complexes which are distinguished by alternating dark and light yellow (coding strand) and dark and light blue (non-coding strand) of the DNA. The crystal is formed of 'infinite' strands of essentially B-form DNA angulated in the eight base pair TATA-box through contact with human TBPc (Figure adapted from Tsai and Sigler, 2000).
As each complex has a different crystal-packing environment, the structure enabled us to identify consistent stereochemical features that are inherent to the complex and independent of the crystal lattice. Our structure shows for the first time that TFIIBc makes base-specific contacts in both the major groove upstream and in the minor groove immediately downstream of the TATA-box. These interactions are sufficient to provide polarity for the nucleating events in the assembly of a transcriptionally competent PIC in the absence of other basal factors.
This work was cited in Advanced Information on the Nobel Prize in Chemistry 2006 by L. Thelander, Member of the Nobel Committee for Chemistry.








