Methods to Explore RNA Processing
The first step to understanding an RBP's roles is to identify the RNAs it interacts with. The development of HITS-CLIP and iCLIP methods revolutionized our ability to map RBP binding sites in vivo by enabling pulldown of an RBP of interest, followed by high-throughput sequencing of crosslinked RNA fragments. However, low efficiency of converting RNA into sequencing library limited their scalability in building large-scale networks incorporating many RBPs. To address this limitation, we and others recently described improved CLIP methodologies that dramatically improve the efficiency of converting immunoprecipitated RNA into high-throughput sequencing libraries, decreasing experimental failures and costs. The improved efficiency in our eCLIP approach enabled quantitative normalization against paired inputs to distinguish true binding events from false positive artifacts, and empowered the generation of 223 eCLIP datasets profiling 150 RBPs in K562 and HepG2 cells.
We are continuing to build upon our eCLIP work to enable profiling of additional RBPs (including those lacking suitable antibodies), as well as in low-input or complex tissue samples. We are also using the eCLIP framework to develop improved targeted methods that deeply explore individual aspects of RNA processing, including deep experimental mapping of microRNA targets and simplified approaches to quantify translation efficiency transcriptome-wide.
Large-scale Maps of RNA Regulatory Networks
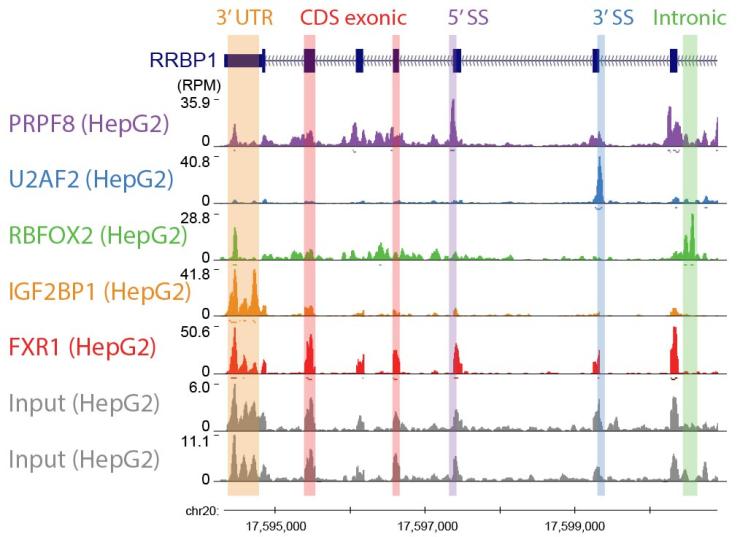
Deep characterization of individual RBPs can give important insights into how an RBP drives molecular and cellular phenotypes. However, layering dozens or hundreds of RBPs together can give further insights by revealing similar and distinct binding modalities and properties between RBPs, identifying co-interacting and co-regulating factors, and identifying critical highly-interacting RNA elements.
Our ENCODE work profiling targets for 150 RBPs revealed insights into basic RNA processing mechanisms in K562 and HepG2 cell lines. In the Van Nostrand lab, we are expanding this effort into RNA viruses (SARS-CoV-2 and Dengue) to build a global regulatory map of host RBP interactions with these viral RNAs, in order to better understand the essential roles host RBPs play in enabling viral replication and infection.
RNA Translation in Breast Cancer
RNA processing has emerged as a critical regulatory step in initiation and progression of cancer, with alterations in RNA transcription, splicing, and translation identified in nearly all cancer types. In particular, alterations in the rate of translation of mRNAs into proteins is a particularly complex yet commonly dysregulated aspect of RNA processing in tumorigenesis, as cancer cells show alterations in both global translation rates as well as differential translation of specific mRNA subsets that drive tumor genesis, progression, and metastasis. However, our understanding of how RBPs can dysregulate RNA translation and contribute to tumorigenesis and progression remains fragmented due to technologic limitations that impede systematic RBP characterizations.
To create the first global map of the landscape of RBPs that drive aberrant translation during emergence and metastasis of breast cancer, we will develop novel integrative approaches to map RNA processing regulatory networks and predict key functional nodes that drive altered translation and tumor phenotypes. In addition to insights into potential therapeutic targets in breast cancer, our development of new techniques and coupled bioinformatic tools will provide a framework for future research into other types of cancer that often share similar aberrations in RNA processing.








