Our projects are question driven and are refined through ongoing discussion within the group. On the surface, some projects may seem far removed from clinical problems, and others, very directly related. Often, very fundamental questions about brain mechanisms are illuminated by clinical and genetic insights. Conversely, lab studies driven by curiosity and unexpected results may reveal previously unknown pathways to new treatments and cures.
Potassium Channels
What do KCNQ2 and related potassium channels do in the brain? In collaboration with Lily Jan and others at UCSF, Dr. Cooper discovered that brain KCNQ2 and KCNQ3 potassium channels were bound in a detergent-insoluble protein complex. With collaborators at Penn, he found this protein complex was located at axonal initial segments and nodes of Ranvier. The initial segments (highlighted in figure below) are tiny sub-domains of the axon where voltage-gated sodium channels spark the action potential, the neuron's fast long-distance signal. Because they are in the axon and so small, these sub-domains are very difficult to study and poorly understood. The team then showed that ankyrin-G, a protein known to bind voltage-gated sodium channels on axons, also bound KCNQ2 and KCNQ3. Surprisingly, the sequence motifs required for this binding of the two unrelated channel types were very similar (see illustration, "anchor" motifs). Ongoing work is exploring the molecular basis of these interactions, the consequences of this binding for axonal function, and the significance of the protein complex for epilepsy and bipolar disorder.
NaV and KCNQ2 co-localization depends on similar ankyrin-G binding "anchor" motifs.
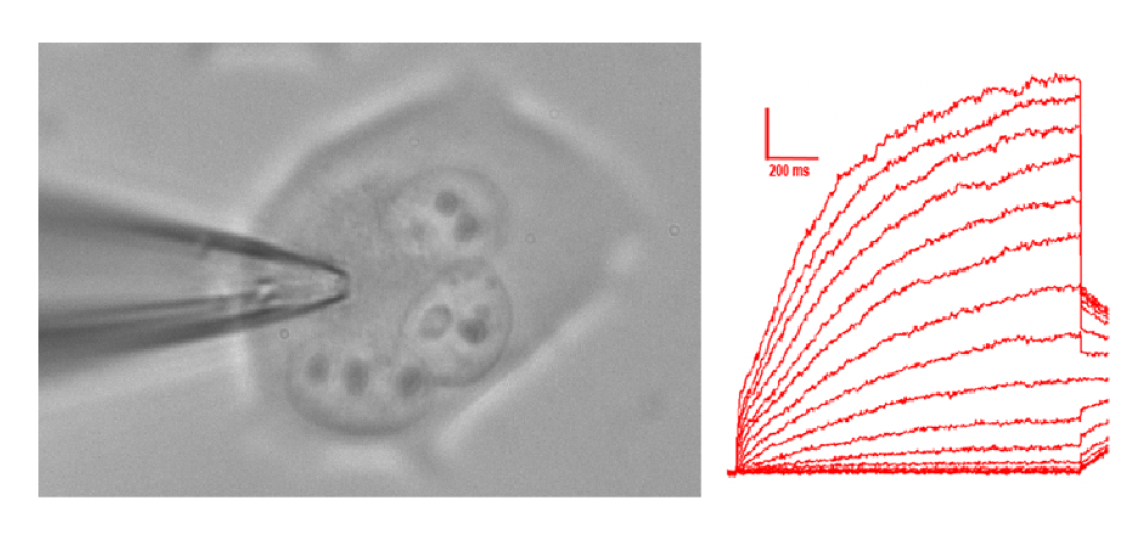
KCNQ2/Q3 Genotype Phenotype Correlations
Why do these channels cause epilepsy that starts shortly after birth and disappears with later development? Why do some variants cause a transient seizures only, but others cause persistent severe limitations in motor, social, and intellectual development even after seizures stop? KCNQ2 and KCNQ3 variants cause benign familiar neonatal seizures (bfns). These variants are passed on in autosomal dominant fashion--affected individuals have seizures that usually begin in the first week of life and remit within a few months. In 2012, novel variants in KCNQ2 were discovered that cause a more severe syndrome in which epilepsy is accompanied by developmental delay ("KCNQ2 encephalopathy"). We are analyzing the reasons for this genotype-phenotype relationship, and the implications for treatment of the persistently affected infants and older individuals. Methods include patch clamp electrophysiology in cultured cells and neurons. Novel approaches are also underway.
Whole cell voltage clamp recording of KCNQ2/3 channels
Post Translational Modifications
How can Post-Translational Modifications of Ion Channels be Comprehensively Monitored? It's cool, and powerful, that rapid openings and closings of ion channels can be captured by electrophysiological recording. Recording can be done in real time, at the level of a single molecule (single channel recording) or together in groups ranging from a few to a few million (whole cell recording). However, each channel is also a crossroads for regulation by and of intracellular pathways. Post-translational modification of the endodomains of channels and associated proteins are one aspect of this conversation between the neuron's interior metabolism and membrane electrical signaling. Such modifications, including phosphorylation and ubiquitinylation, are thought to be a ubiquitous mechanism for rapid-onset, reversible, but potentially durable modulation in ion channel function. Nonetheless, they are very incompletely understood. We have worked to develop multidimensional approaches combining traditional biochemical and newer mass spectrometry approaches to measure changes in modification state underlying neuromodulation, dynamically and quantitatively.
Detection of a KCNQ3 phosphorylation site by affinity purification and LC/MS/MS.
Ion Channels and Evolution
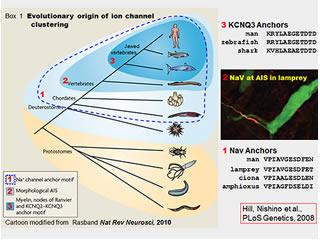
How did the "excitozone" emerge through evolution? What role did this play in the divergence, ecological strategy, and resilience of the vertebrates?
We found that the ion channel protein complex assembled by Ankyrin-G at mammalian axon initial segments and nodes of Ranvier is also present at the axon initial segments of lamprey eels (figure, right). Lampreys, the most ancient of living vertebrates, evolved nearly 500 million years ago. This is about 50 million years before vertebrates with myelinated axon first appeared. Our findings suggest that ankyrin-G dependent clustering of sodium channels via anchor motifs evolved even earlier, however, in small wormlike creatures that are the closest invertebrate relatives of the vertebrates (see figure, right). We are studying these organisms, the basal chordates, to understand how this molecular and cellular innovation emerged. These organisms offer many technical experimental advantages, but the work also is of broader, even philosophical interest. Evolution is often described as a series of changes in visible body shape (Darwin's "endless forms most beautiful"). In this case it appears that an inconspicuous molecular innovation, allowing the individual neuron to signal more rapidly and reliably, may have preceded and made possible the larger brains, larger bodies, and particular forms of complex behavior characteristic of vertebrates. This is a grand and idealistic notion, and an old one--as per the German playwright, poet, philosopher and historian Friedrich Schiller (1759-1805): "it is the mind, itself, which builds the body". (Thanks to Tom Schwarz and Jeffrey Hamburger for sharing the Schiller quote-carved in stone at Harvard).








 Credit
Credit