Identification of periosteal skeletal stem cells and their function during bone development and injury healing.
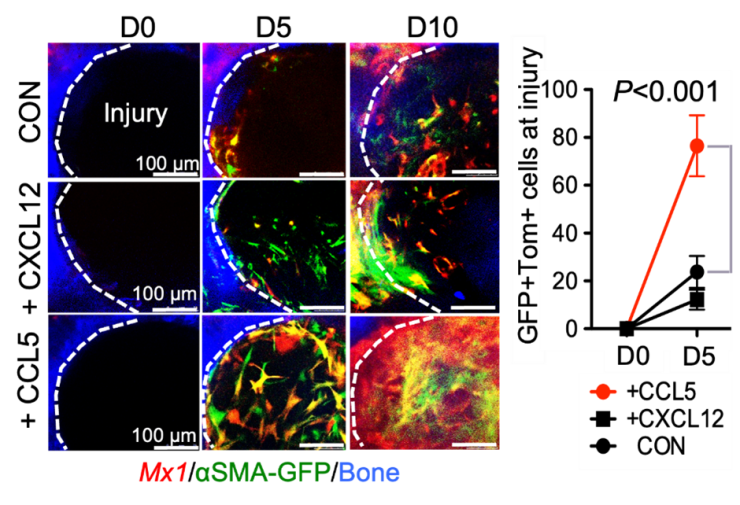
Skeletal stem cells (SSCs) are heterogeneous in population and have multipotent capacity to differentiate into bone, adipocyte, cartilage and marrow stromal cells. In particular, they continuously supply chondrocytes and osteoblasts for cartilage and bone formation, respectively, during skeletal development and bone regeneration/repair process throughout lifetime. In particular, in vivo identity and specific regulatory mechanism of adult periosteum-resident SSCs are unknown and how they are different from bone marrow resident SSCs. Our lab has previously identified functionally distinct P-SSCs using a combination of Mx1 and SMA to selectively label resident P-SSCs in adult periosteum. These P-SSCS specifically express the CCL5 receptor CCR5 and CCL5 induces in vivo migration and bone healing, while loss of CCL5 delays bone healing.
To further characterize the selective markers to distinguish different subsets of SSCs in vivo and to improve our understanding in SSCs, our lab investigates the cellular composition and heterogeneity of both the human and mouse periosteum by single-cell analysis to define the molecular signatures of endogenous SSCs and how they are different from bone marrow resident SSCs. Moreover, we study their functions during bone development and bone injury healing response by using cutting edge techniques, such as intravital imaging of living mice to trace the fluorescently labeled stem cells and how they change over time.
Differential Regulation of Calvarial vs Long Bone Skeletal Stem Cells in Bone Homeostasis and Osteogenesis Imperfecta
Reconstruction of bone defects is important but challenging in patients with Osteogenesis Imperfecta (OI) or deformities in craniomaxillofacial (CMF) regions. Recently, calvarial suture skeletal stem cells (SuSSCs) were identified as a main stem cell population for adult CMF bone regeneration. While much is known about skeletal stem cells in long bones, how the molecular and regulatory mechanisms of SuSSCs are different from long bone SSCs during skeletal development and injury healing have not yet been well elucidated. Further, their differences in normal and OI conditions are essentially unknown. By comparing SuSSCs with long bone SSCs, we aimed to identify the differential function and molecular signatures during postnatal skeletal development and OI bone regeneration. Our initial findings showed that SuSSCs in calvaria injury have delayed migration and delayed healing in OI mice. Single-cell analysis further revealed that calvaria and long bones have unique stem/progenitor clusters which we are currently investigating.
Bone marrow stromal cell regulation of medullary cavity formation and maintenance.
Bone marrow stromal cells are a major source of bone marrow (BM) osteoblast and adipocytes, of which adipocytes are reported to negatively impact bone health. Nearly all bone marrow adipocytes originate from early postnatal BM stromal cells (BMSCs), however ~80% of these cells do not differentiate to mature lipid laden adipocytes nor do they differentiate to osteoblasts in young or older mice. Why these BMSC subsets appear in early BM and which populations and signals prevent bone formation in the medullary cavity are unknown. Ablation of these BMSCs drastically increased medullary cavity bone mass. Additionally, they are injury responsive and localize to the inner fracture callus during healing, indicating these BMSCs have a distinct anti-osteogenic function within medullary cavities. The main objective of this project is to define the in vivo role of a unique BMSC subset in bone medullary cavity maintenance and bone repair.
Novel tendon stem/progenitor cells and their signaling for postnatal tendon growth and repair
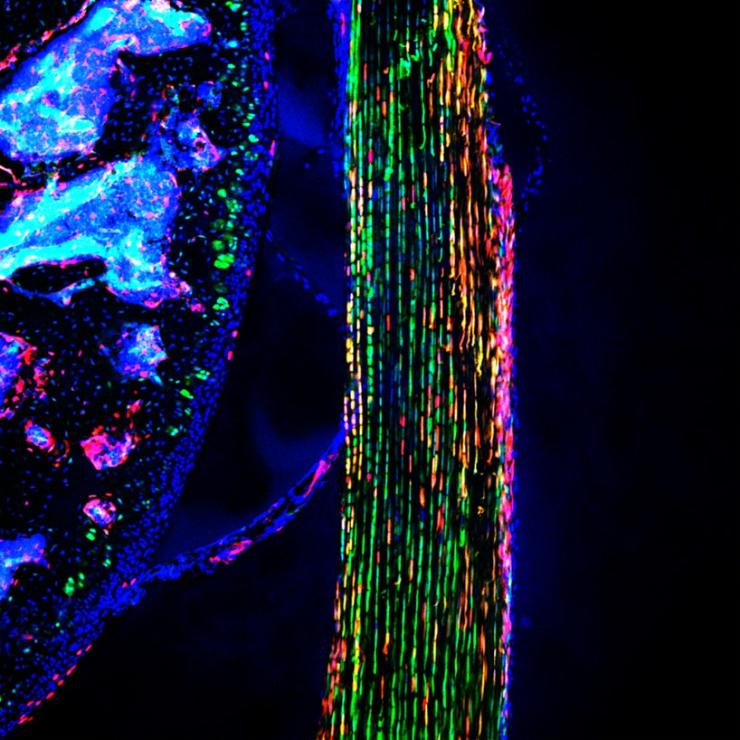
Tendon and ligament injuries are the most common types of sport injuries, but incomplete healing, sustained pain and scars, and frequent re-ruptures even after surgical treatments remain a major clinical challenge. In particular, the in vivo identity and regulatory mechanism of tendon stem/progenitor cells (TSPCs) responsible for adult tendon regeneration and repair are largely unknown. By using single cell analysis and intravital imaging of new TSPC animal models, we found that a distinct TSPC subset newly appears in postnatal paratenon layer and continuously contribute to adult tendon growth. Sequential in vivo imaging revealed that TSPCs have perivascular location in normal tendon and upon injury, they migrate to the injured sites, proliferate, and differentiate into new tendon cells. Further, these TSPCs sustain their population and continuously supply the majority of new tendon cells even after multiple rounds of injury. We are now studying their molecular regulatory mechanisms to preserve progenitor function and differentiate into mature tendon cells.
Identification of pulp progenitor cells as contributors to postnatal odontoblasts and mature pulp cells
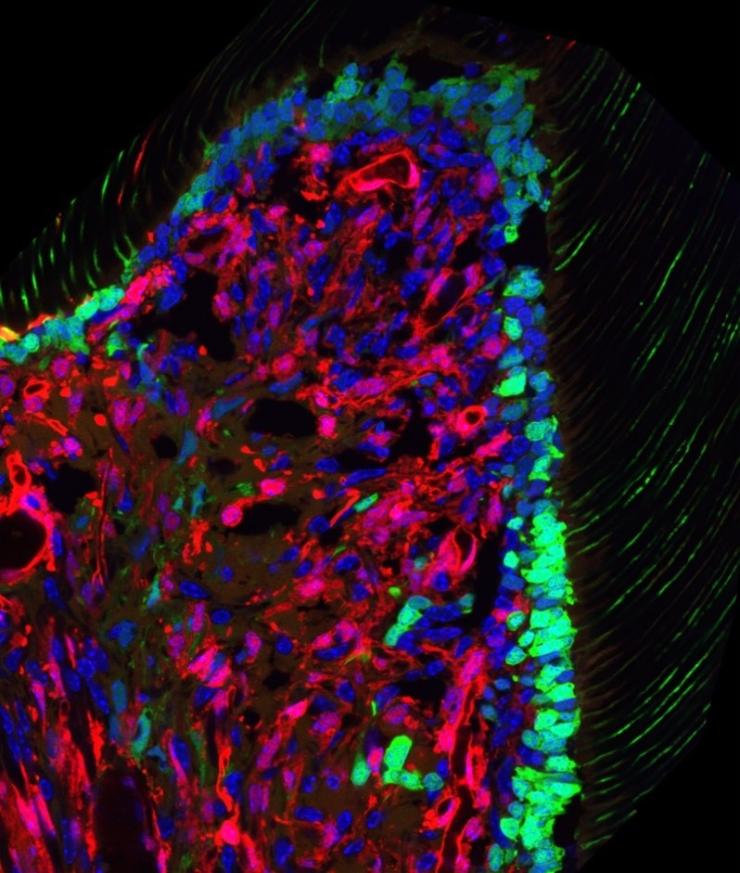
Regeneration of dentin and odontoblasts from dental pulp stem cells (DPSCs) is essential for permanent tooth maintenance. However, the in vivo identity of endogenous DPSCs and their role in reparative dentinogenesis are elusive. We used pulp single-cell analysis before and after molar eruption and revealed that endogenous DPSCs in coronal papilla are long-term repopulating cells contributing to the majority of pulp cells and new odontoblasts. Upon molar injury, DPSCs localize into the injury site and differentiate into new odontoblasts, forming unique dentinal tubules and reparative dentin. We are currently working on single-cell and FACS analysis to define their stem cell marker expression and upstream signals for odontoblast differentiation.








