Could tissue sparing surgery get rid of that pain in the neck?
Despite being one of the most common spinal surgeries, anterior cervical discectomy and fusion, a type of treatment for forms of degenerative disc disease, is a major procedure that sometimes fails in certain high-risk patients and situations. Doctors with Baylor College of Medicine and Baylor Medicine’s Spine Center, as well as Baylor St. Luke’s Medical Center are now part of the FUSE study, a multicenter clinical trial working to find a tissue-sparing approach to increase the success rate of neck fusion surgery to treat nerve compression and neck pain.
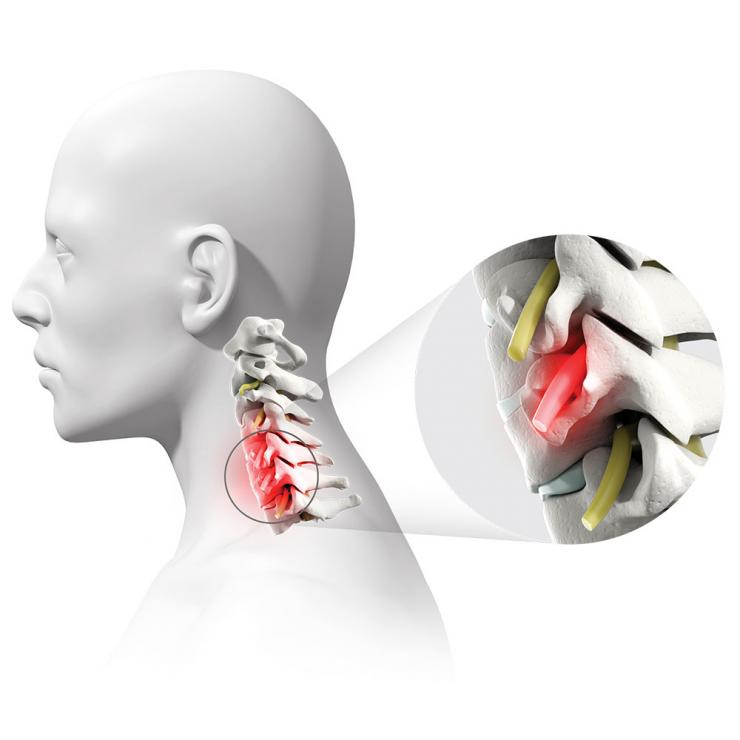
Degenerative disc disease is a condition that causes pain because the discs that usually provide cushion in the spine have worn away due to age or injury. The pain can radiate down the neck, shoulder and arms. Sometimes, bone spurs or a disc herniation can result, causing nerve or spinal cord compression that can be disabling. The common treatment is to remove the disc and bone spurs from the front of the neck and then have the two adjacent vertebrae fuse together – an anterior cervical discectomy and fusion (ACDF).
“An ACDF decompresses the spine, then permanently fuses the bones together to reduce continued degeneration,” said Dr. David Xu, assistant professor of neurosurgery at Baylor and surgeon with Baylor St. Luke’s Medical Center. “The surgery requires a small incision through the front of the neck and is successful more than 95% of the time when treating a single level of the vertebra.”
However, when treating high-risk patients such as those with diabetes, kidney disease or osteoporosis, or when needing to fuse multiple levels, failure is more common. According to Xu, “fusion failure can sometimes exceed 20 percent when three or more discs need treatment.”
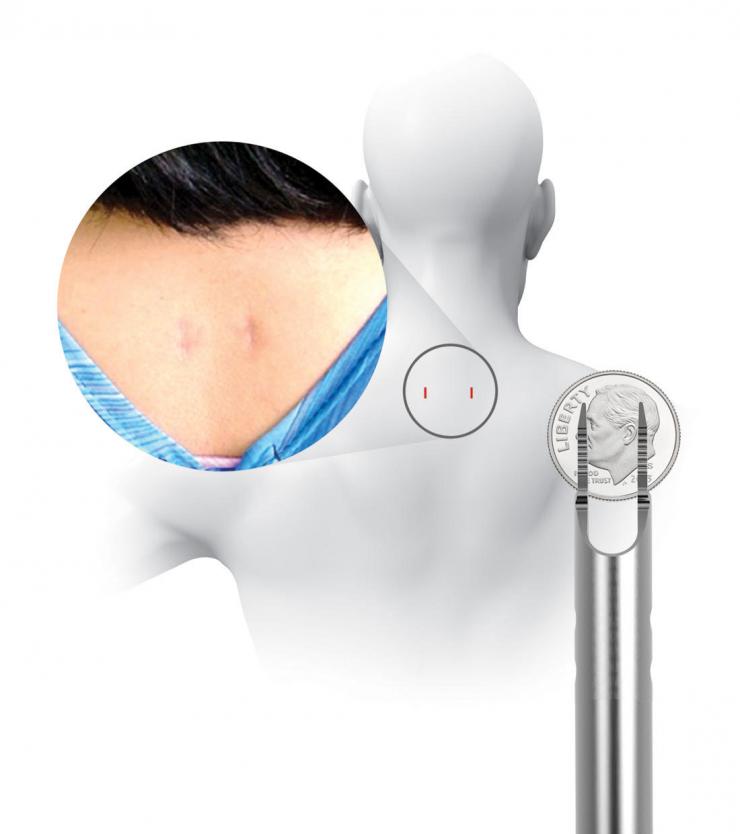
Traditionally, the way to prevent or treat fusion failure is to supplement the ACDF by making a big incision in the back of the neck and installing screws and rods, resulting in the disruption of muscles and soft tissues. In the current study, a unique set of implants are used to achieve the same supplemental stabilization without the big incision. The devices are inserted into the back of the neck through two small one-centimeter incisions using a unique set of instruments called the CORUS Spinal System. The process is tissue-sparing and drastically reduces muscle disruption and blood loss.
The Fuse Trial is a prospective, randomized, controlled study, which means participants will be randomly chosen to receive ACDF alone or ACDF plus supplemental stabilization with posterior cervical stabilization system (PCSS). The trial will determine if ACDF plus PCSS will result in superior clinical outcomes versus ACDF alone in the treatment of 3-level cervical degenerative disease.
“The goal of this study is to find the most effective method for positive outcomes in high-risk patients, specifically for ACDFs spanning three levels,” Xu said. “We want to help create the gold standard for this type of surgery, making it easier for patients to recover with minimal damage to the muscles in the neck.”
The study is seeking to enroll 330 patients. Participants should be between 18 to 80 years of age and have been recommended for ACDF treatment for degenerative disc disease between and including the C3-C7 vertebrae. The study is sponsored by Providence Medical Technology.
For a full list of study locations and eligibility criteria, see clinicaltrials.gov.







 Credit
Credit