Gastrointestinal Focus
Therapeutic probiotics are promising agents for microbiome manipulation, and could be applied as a means to prevent or treat a variety of pediatric illnesses ranging from acute infections to chronic immune disease. Rational selection of such agents will aid in the expansion of safer treatment options for children. Dr. Spinler has developed techniques that combine bacterial genetics, biochemistry, and functional genomics to characterize probiotic anti-infective mediators generated by Lactobacillus reuteri.
L. reuteri is a beneficial microbe indigenous to humans and other vertebrates that has the potential to influence neighboring microbial communities. Through a combination of comparative genomics and functional analyses, we have shown that human strains from two distinct ecotypes, clades II and VI, differ markedly in bacterial functions that are likely to influence the interrelationships of these strains with the human host (Fig 1) (Spinler, et al. 2014, Genome Biol. Evol. 6(7):1772). Human-derived L. reuteri strains ferment glycerol in a vitamin B12-dependent manner to produce a broad-spectrum antimicrobial compound, reuterin. In addition to reuterin production, L. reuteri carries out de novo synthesis of vitamin B12, serving to benefit both the microbe and the host. Clade VI strains, in particular, produce greater amounts of reuterin than clade II strains and may be better suited for the treatment or prevention of gastrointestinal tract infections.
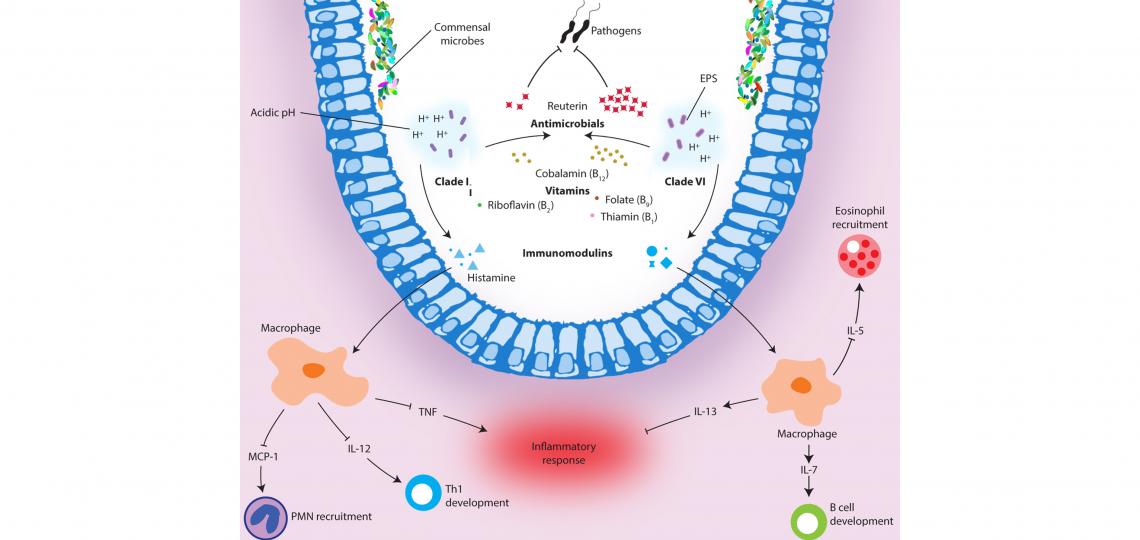
Figure 1. Functional illustration of human-derived Lactobacillus reuteri ecotypes. L. reuteri strains from both clades modulate acidic environments, produce the antimicrobial reuterin, synthesize essential vitamins, and generate immunomodulatory compounds that effect immune signaling in the host. The differences associated with each ecotype are illustrated here. Spinler, et al. 2014. Genome Biol. Evol. 6(7):1772-1789.
Building on these studies, we have selected a clade VI L. reuteri strain as a potential preventative therapy against Clostridium difficile infection (CDI). C. difficile pathogenesis occurs when the normal gut microbiota is disrupted by antibiotic use and it is well known that restoration of intact intestinal microbiota is most successful in preventing severe, recurrent CDI. Metagenomic data analysis of stool specimens from adults with CDI show that the relative abundance of Lactobacillus spp. is markedly decreased and highly predictive of disease recurrence. Also, Lactobacillus spp. are most abundant in children who are generally refractive to CDI. There is a major gap in our understanding of how host bacteria protect against C. difficile, and these probiotic features (antimicrobial production and vitamin synthesis) may work in concert to allow L. reuteri to mold the local microbial community by antagonizing the growth of pathogenic organisms and selecting for the growth of more favorable microbes, all the while enhancing the health of the host.








