Identifying Molecular Translators in Embryonic Axis Extension
Patterning cues along the anteroposterior embryonic axis are required for extension of that axis, but how is this coordination achieved at the molecular level? We are working to identify ‘molecular translators’ that allow complex spatial cues to be interpreted by an embryo’s morphogenetic machinery that shapes the nascent body plan.
Origins of Asymmetry Underlying Extension Morphogenesis
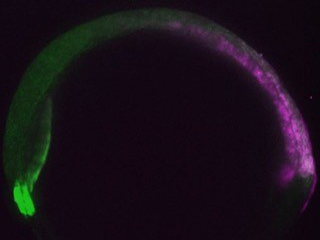
Asymmetry along the head-tail axis is required for its extension. Such asymmetry is apparent within zebrafish embryo explants, which recapitulate axial extension in culture, but how it arises is unknown. Is it simply imposed by the application of exogenous signaling molecules? Or is an intrinsic asymmetry ‘baked in’ to these cells? What are the underlying molecular mechanisms in either case?
Timing of Morphogenesis
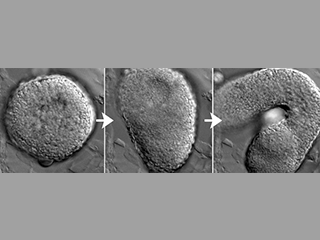
Axial extension begins exactly on time in embryo explants, despite their isolation from endogenous embryonic cues. This implies that these cells possess an internal ‘morphogenetic clock’. We want to know, what is the molecular basis of this clock? And how does it set morphogenesis is motion?
Molecular Regulators of the Migratory Switch
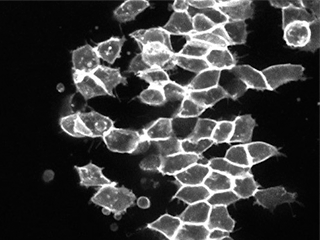
Invasive cell migration is a cell behavior program required for normal embryonic development, but is inappropriately reactivated during cancer metastasis. By tuning signaling levels within our embryonic explants, we can drive cells into either migratory (invasive) or non-migratory states in culture. Using this simplified platform, we aim to characterize the molecular landscapes that promote – or inhibit – this cellular migratory switch during development and disease.








