Acquire the knowledge and skills you need to break barriers in cancer and cell biology.
About the Program
The Cancer & Cell Biology Program provides broad, interdisciplinary training in the fundamentals of normal cell function and cancer, emphasizing a wide spectrum of genomic analyses to growth, invasion and metastasis.
Most of our faculty members are members of BCM’s National Cancer Institute-designated comprehensive cancer center, the Dan L Duncan Comprehensive Cancer Center. Many are also members of the BCM Department of Molecular and Cellular Biology, which is ranked in the top five in the country for National Institute of Health funding. Collaboration is facilitated through multidisciplinary centers focused on cell and gene therapy, stem cell and regeneration and aging, which enhance the highly collaborative and interactive environment.
You will train with faculty members who have a prestigious record of achievement in advancing understanding of gene regulation, hormone action and cell signaling, genomics, proteomics, metabolomics, cancer-related immune therapies and reproductive medicine.
Our Program
We have incorporated the best elements of traditional graduate programs – academic rigor and stellar faculty – with flexibility that supports intensive academic training in small group formats while providing you the freedom and support necessary to design an individualized curriculum.
Where Will Your Ph.D. Take You?
Our program will give you the background and expertise needed to succeed in your scientific and professional career. Whatever your vision for your career entails, we will provide the training, resources and support to help you realize your ambition.
Cancer & Cell Biology News
Losing neurofibromin is good news for breast cancer
It is well known that neurofibromin (NF1), a tumor suppressor produced by the NF1 gene, keeps cancer growth in check by repressing the activity of a cancer driver gene called Ras. It then follows that when NF1 is lost, Ras can drive cancer growth by promoting treatment resistance and metastasis. NF1, however, can do more than regulate Ras. BCM researchers and their colleagues have discovered new insights into the function of neurofibromin that improve our understanding of breast cancer resistance and suggest novel therapeutic approaches to overcome it.
AKAP8 and the delicate balance between normal and metastatic states
A protein naturally produced in the body has been found to suppress breast cancer metastasis in animal models of human tumors. In this study, the same team at BCM that published a pioneering study that brought alternative splicing to the field of cancer research, investigated how alternative splicing contributes to cancer metastasis by looking for proteins that regulate alternative splicing events linked to metastasis.
Like a mastermind of its own destiny, glioma sets a stage that favors its own growth
BCM researchers and colleagues uncovered evidence about how glioma manipulates its environment in ways that favor its own growth. This research, published in Nature, suggests new strategies to treat patients with this condition, one of the most aggressive malignant primary brain tumors.
An unprecedented look at endometrial cancer
Which therapy will benefit this particular patient the most? BCM researchers and colleagues offer insights that might help physicians better answer this question for endometrial cancer, commonly known as uterine cancer.
Scientists get up close and personal with breast cancer
Analysis of proteogenomics data enables scientists to get up close and personal with cancer and more likely to be able to identify tumor weaknesses that may make it susceptible to specific therapies. Researchers have not been able to apply proteogenonics analysis to cancer diagnosis in the clinic simply because the size of the tissue sample required for this type of analysis is larger than the biopsy sample usually obtained for diagnosis. This study describes methods developed that enable detailed analyses of high-quality DNA, RNA and proteins from a single core needle biopsy.
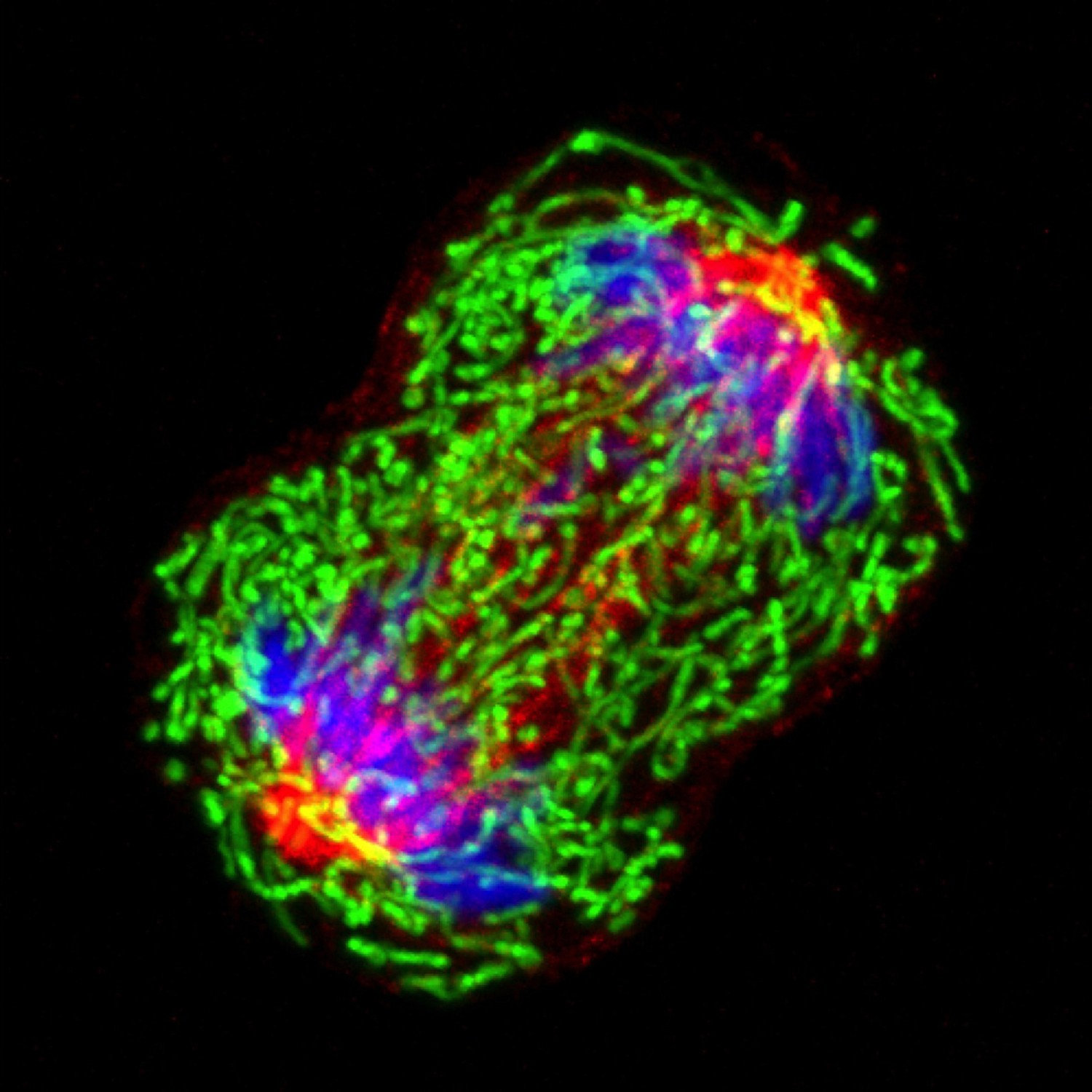
Not in sync: single cells and genes show unanticipated response to estrogen
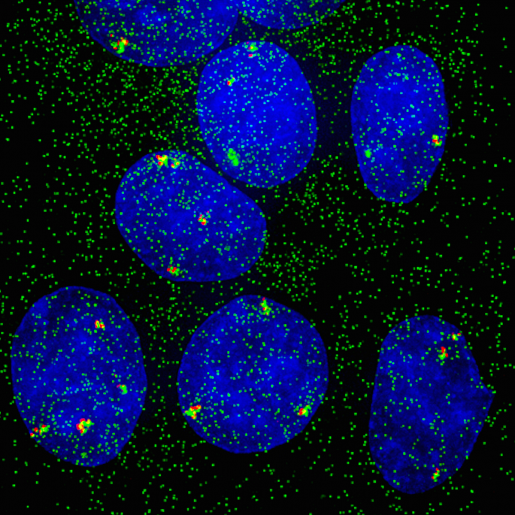
When experimental findings are not compatible with the textbook view, scientists know they have quite a challenge in their hands. BCM researchers and their colleagues faced this situation when they were investigating how cells modulate their responses to estrogen at single-cell and -gene level. In the current study, researchers worked with human breast cancer cell lines grown in the lab. Using both molecular and imaging analyses, they determined, at single-cell and -allele levels, the expression of two well-characterized genes whose activity is regulated by estrogen.
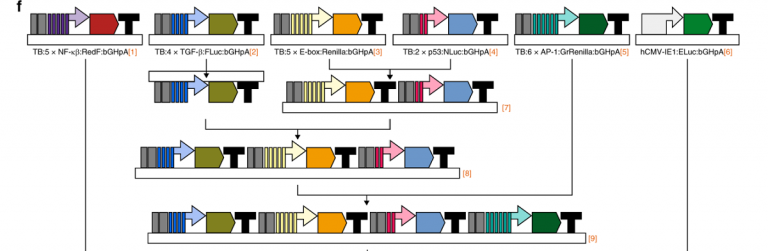
Six is better than two: assay assesses multiple cellular pathways at once
To get a detailed picture of the cellular processes that differentiate normal versus cancer cells, researchers resort to conduct several independent screening assays at the expense of time and additional cost. In this study, the goal of the BCM researchers was to measure multiple cellular pathways at once in a single biological sample, which would also minimize experimental errors resulting from conducting multiple separate assays using different samples.
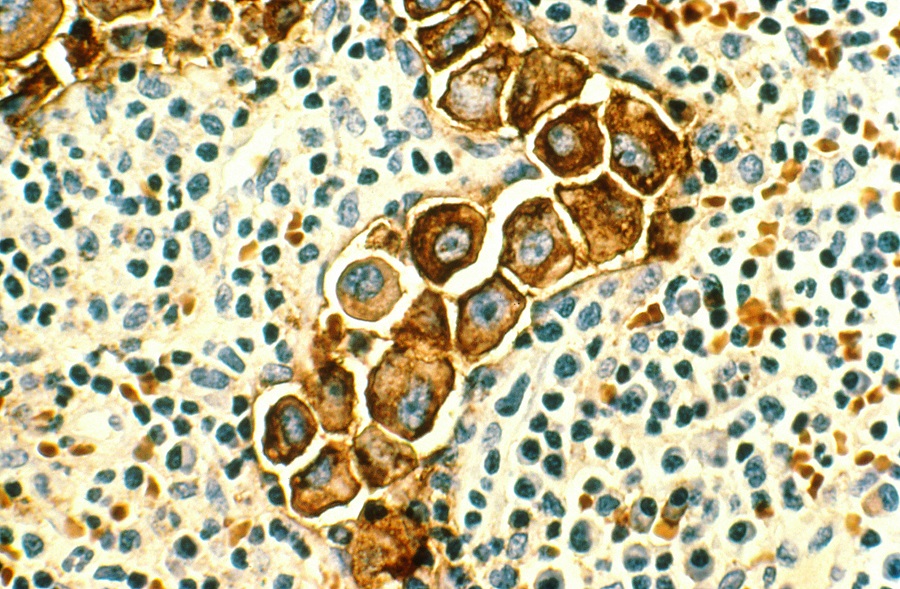
Becoming metastatic: how FOXA1 triggers metastatic behavior in breast cancer
About 75 percent of breast cancers have estrogen receptors, hence they are called estrogen receptor positive (ER+). Original ER+ breast cancer cells depend on estrogen to grow, and therapies that make estrogen unavailable to cells, called hormone therapies, can result in long-term remission in some patients. However, most patients with metastatic disease, including those whose tumors responded initially to hormone therapy, eventually relapse and die due to the tumors’ acquired resistance to hormone therapy. BCM researchers and colleagues unveiled a novel mechanism that helps explain how endocrine-resistant breast cancer acquires metastatic behavior, opening the possibility of new therapeutic strategies.

From the Labs
Keep up-to-date on all the research news from Baylor College of Medicine. Subscribe to our From the Labs blog.
Training Grant
The Cancer & Cell Biology Program is supported by National Institute of General Medical Sciences Ruth L. Kirschstein National Research Service Award (NRSA) Predoctoral Institutional Research Training Grant (T32) Training Grant GM136560.
In order to earn this grant, our program successfully demonstrated that we provide high-quality research training, mentored research experiences, and additional training opportunities that equip trainees with the technical (e.g., appropriate methods, technologies, and quantitative/computational approaches), operational (e.g., independent knowledge acquisition, rigorous experimental design, and interpretation of data) and professional (e.g. management, leadership, communication, and teamwork) skills required for careers in the biomedical research workforce (i.e., the breadth of careers that sustain biomedical research in areas that are relevant to the NIH mission).
Stipends and Benefits
At BCM we are focused on you and your training. If your vision for your future includes teaching, you may choose to gain experience as a teaching assistant. If you do not want to teach, you have the freedom to focus exclusively on your education and research as well as to work with your mentor to take advantage of other BCM resources that match your interests.
Graduate School of Biomedical Sciences
The Cancer & Cell Biology graduate program is part of the Baylor College of Medicine Graduate School of Biomedical Sciences (GSBS). Explore the full GSBS website for more information about our curriculum and admissions process as well as to find resources and services designed to support your success throughout graduate school and your future career.
Dan L Duncan Comprehensive Cancer Center
A couple of years ago, BCM's cancer center earned designation as a Comprehensive Cancer Center from the National Cancer Institute. Dr. Kent Osbourne discusses the depth and breadth of research and clinical initiatives within the Center that made this achievement possible.











