The Center for Precision Medicine Models at Baylor College of Medicine accepts submissions from clinicians, genetic counselors, and researchers who seek to demonstrate the functional impact of a variant of interest through the development of model organisms. All variants proposed for modeling will be reviewed by the Baylor Center for Precision Medicine Models Variant Review Committee following submission of the Baylor CPMM Variant Submission Form.
Candidate variants will be evaluated based upon a broad set of criteria, including:
- Suitability for modeling by our center
- Novelty of the variant, potential impact of the clinical question that may be addressed by modeling
- Quality and completeness of information associated with the variant
- Willingness of the applicant to communicate and collaboratively engage with Baylor CPMM team
Variant Evaluation Criteria
The Baylor CPMM-VRC carefully evaluates all submitted variants for suitability for precision animal modeling (mouse and fly) within our center. The center will also evaluate our large database of genome and exome data from rhesus macaques to identify potential non-human primate models with spontaneous mutations relevant to the submitted variant.
Variants that may be of most interest to our center include those that have the potential for:
- Illuminating the mechanism of variant pathogenicity to guide prognostic or genetic counseling
- Providing new insights into variant pathogenicity to guide therapy
- Generating experimental evidence to establish a potential novel disease gene
- Evaluating utility of putative biomarkers for disease diagnosis and monitoring
- Facilitating evaluation of phenotypic expansion and/or need for phenotypic surveillance (for preventive medicine) by performing deep-phenotyping of models generated for the variant
- Developing a pre-clinical model for potential use in future therapeutic studies
Additionally, there may be variant submissions that do not meet our scientific or medical threshold for modeling.
Variants that may not be suitable for our center are those focused on:
- Assessing the function of a VUS (variant of uncertain significance) in a known disease-causing gene when the patient phenotype is consistent with known disease phenotype for purely diagnostic purposes (no plans to follow up on pathogenic mechanisms)
- Animal models for polygenic diseases that require manipulation of more than two genes
- Genetic diseases with low penetrance or expressivity
- Modeling diseases that have a strong environmental component that may be difficult to model in non-human studies
The examples provided above are illustrative only and should not be considered exhaustive. For additional data on submitted, rejected, and accepted variants, please visit Variant Submission Data.
Variant Submission and Review
- Variant are submitted via the Baylor CPMM Variant Submission Form.
- After submission, you will receive an email with instructions after we have reviewed your form for completeness.
- You will receive a confirmation email after we have reviewed your form for completeness. We may request clarification of submitted information or additional information necessary for our review.
Important: Please do not include HIPAA-protected information in your submission.
Each submitted variant will be carefully reviewed for suitability for modeling with our center by the Baylor CPMM-VRC. Once this evaluation process is complete, we will inform the submitter of the results of our preliminary evaluation. Our goal is to complete the evaluation process within six weeks of nomination. In some cases, the submitter may be invited to a Baylor CPMM meeting to discuss the variant and modeling plan. Submitted information, review status, reviewer comments, and a decision letter will be accessible through our Submission Tracking System.
Thank you for your interest in working with Baylor CPMM!
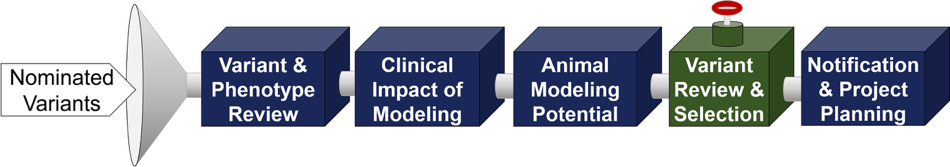
Process for Submitted Variants
- Variant and phenotype review
- Clinical impact of modeling
- Animal modeling potential
- Variant review and selection
- Notification and project planning








